Hydrogen Permeation Measuring Station
H2PU – Hydrogen Permeation Unit
Hydrogen uptake and permeation during electrochemical processes is a critical effect on the mechanical stability of some metals, especially iron- and copper-based materials.
Together with ICA, we are launching a complete measurement system for the determination of hydrogen permeation, now.
Today, production- and operation-related material embrittlement caused by hydrogen confronts designers and process operators with unexpected challenges.
Starting point for hydrogen-induced material embrittlement can be coating processes and their pre-treatment as well as corrosion processes in component operation under mechanical load.
In recent years, the measurement method of hydrogen permeation has become established for the investigation of phase boundary reactions with special consideration of hydrogen formation.
Existing measuring systems are characterised by a complex setup according to Devanathan and Stachurski.
Together with ICA, we are launching a complete and robust measuring system for the determination of hydrogen permeation, which performs its electrochemical measuring service without the use of expensive potentiostats.
When examining steel foils, it is also possible without Pd coating on the oxidation side of the sample.
In doing so, you can perform a wide range of electrochemical measurement and control tasks:
- In situ monitoring of hydrogen evolution during electrochemical coating, including monitoring of the potential at the phase boundary.
- In situ monitoring of hydrogen evolution in pretreatment processes, such as pickling processes, in conjunction with monitoring of the electrochemical potential at the phase boundary.
- Monitoring of production process parameters with regards to hydrogen embrittlement.
- Determination of material embrittlement during transport and storage of hydrogen in tank lines and storage systems.
- Determination of H-diffusion coefficients in metallic materials in dependence of the morphological material properties.
However, the measuring system is not only suitable for investigating hydrogen permeation, but can be used in all areas of electrochemistry:
- Investigation / simulation of corrosion reactions.
- Development of electroplating baths and monitoring of deposition kinetics (bath efficiency/current yield).
- Development and evaluation of products for electrochemical processes.
- Setup of complex laboratory experiments with high-resolution measurement electronics.
The measuring system consists of the ElyFlow test cell from Gaskatel and the Automated Measurement and Control Box (AMB) from iChemAnalytics GmbH.
The cell components of the ElyFlow measuring cell are made of PTFE and therefore indestructible.
CNC technology enables precise and reproducible production of all relevant bores, recesses for seals etc.
The Haber-Luggin capillaries end close to the working surface. This means that the potential is not disturbed by the electrical field or by any gas bubbles that are created.
The voltage drop across the electrolyte is very low due to the small distance.
The working surface of the electrode is 10 cm2.

ElyFlow – The electrochemical tet cell with three-electrode setup for your electrochemical measurements.
The Automated Measurement and Control Box (AMB ) of iChemAnalytics GmbHiChemAnalytics GmbH enables the reproducible control and measurement of electrochemical processes with high-resolution measurement technology and simple operation.
The user interface allows intuitive configuration of the inputs and outputs within a few minutes.
The data can be viewed in the live view of the software


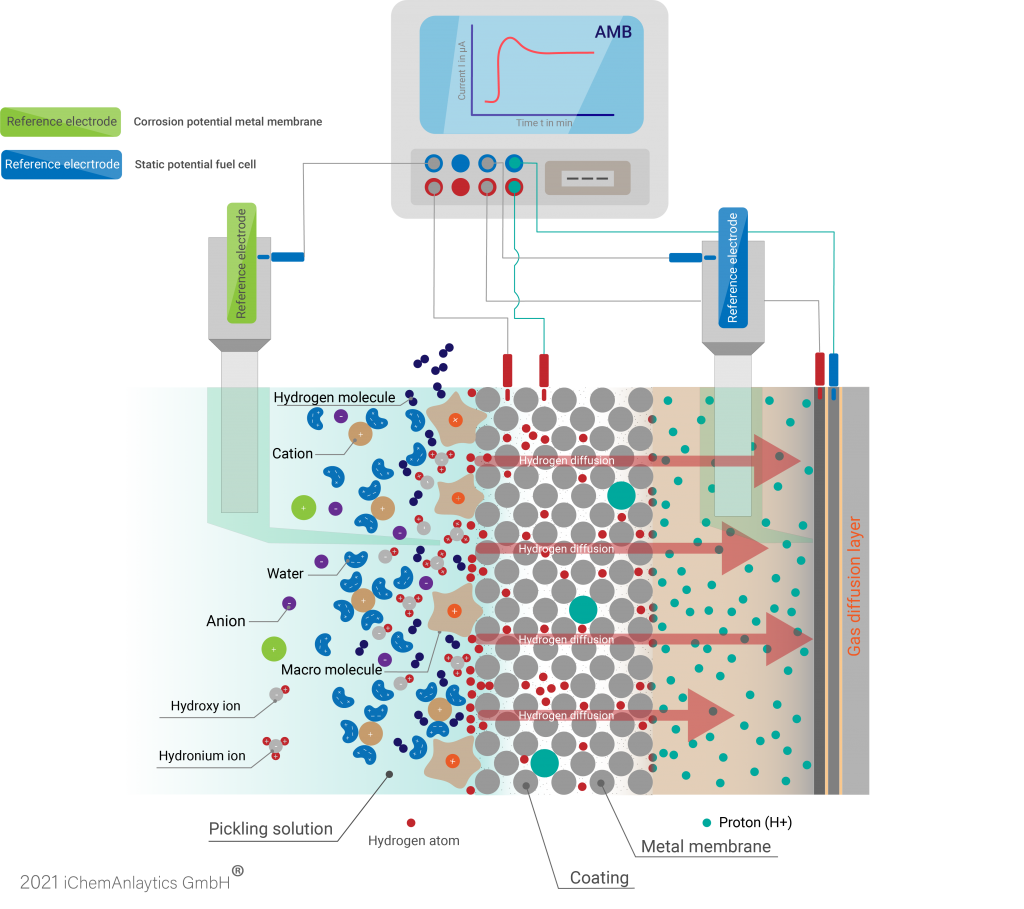
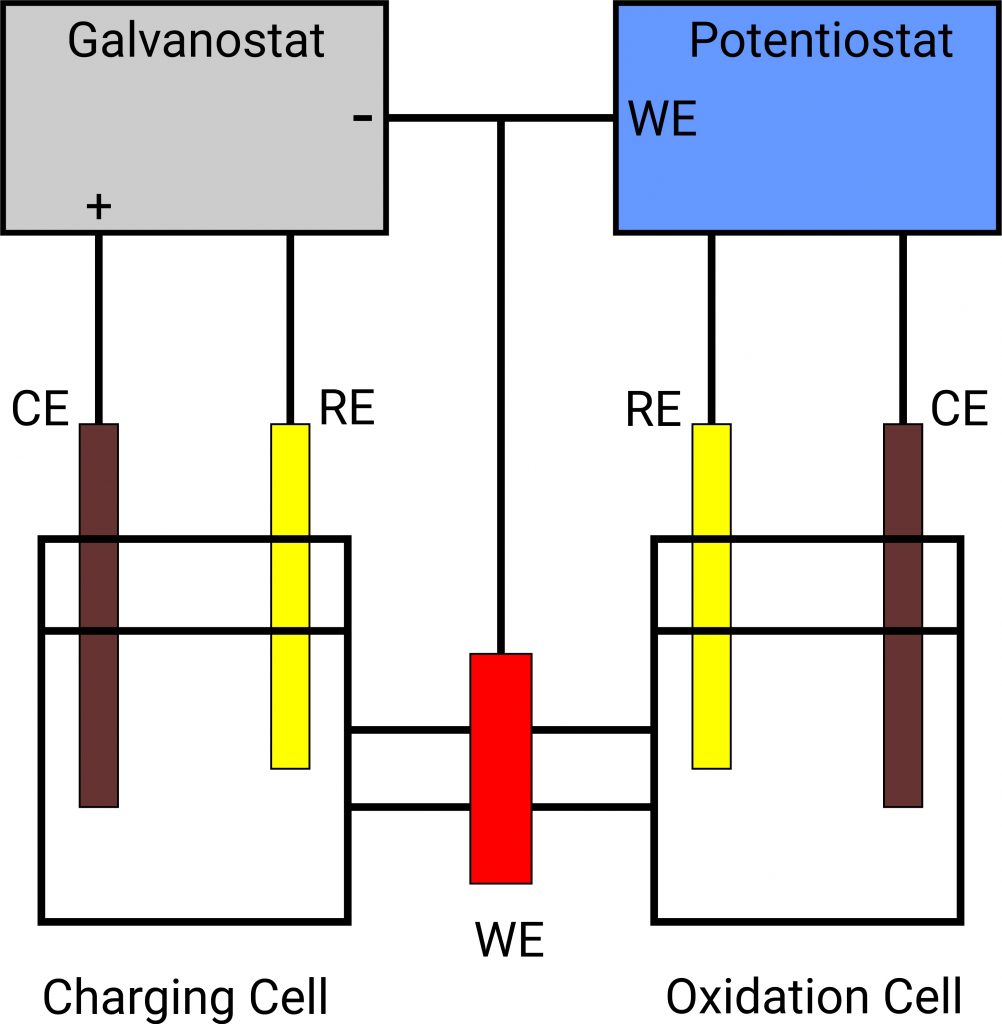
Permeation measurement according to Devanathan and Stachurski
In order to study the diffusion of hydrogen into metals, Devanathan and Stachurski developed the electrochemical permeation technique.
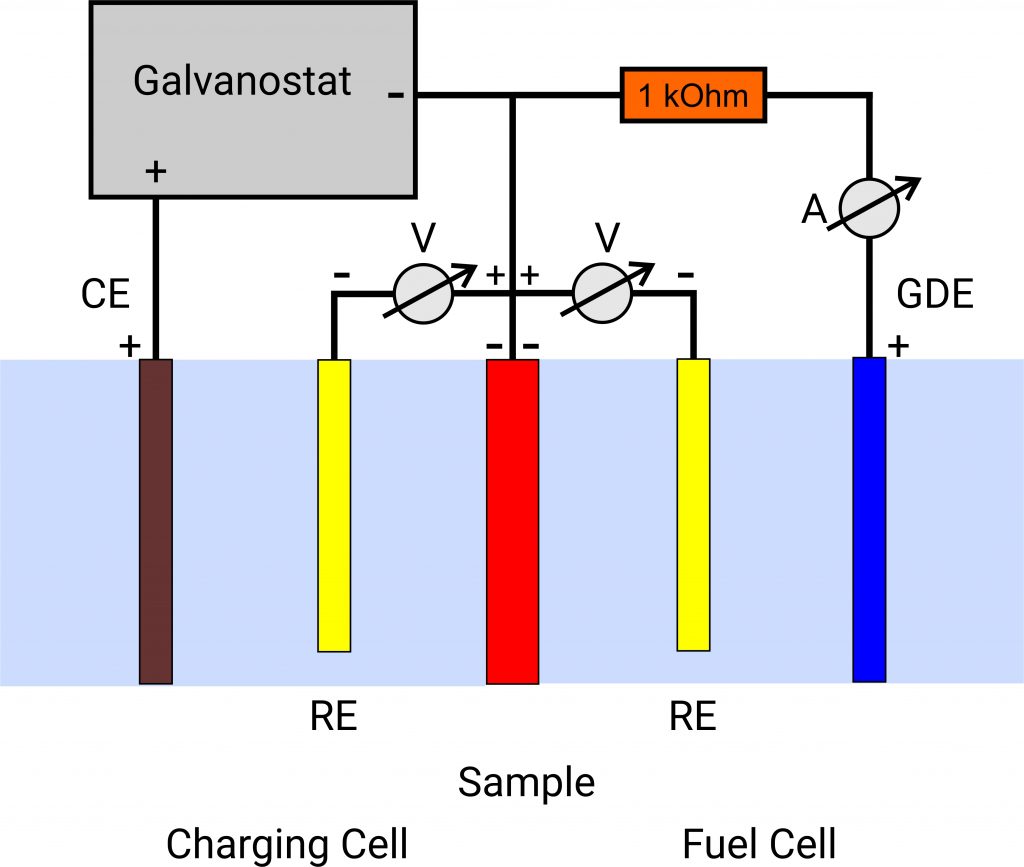
In the charge cell, hydrogen is generated at the sample (working electrode AE), some of which escapes as gas. The remaining part of the hydrogen adsorbs on the membrane and diffuses through it. In the second circuit, the membrane sample is polarised so that the hydrogen is immediately oxidised at the outlet side (oxidation cell). The current flowing during this process is measured. From this, the hydrogen permeation and corresponding diffusion coefficients can be calculated.
For this arrangement you need a potentiostat as well as a galvanostat.
Easy measurement of hydrogen permeation with the H2PU
Our concept, on the other hand, is characterised by less technical equipment.
The sample to be analysed is installed as working electrode between two ElyFlow analysis chambers.
The counter electrode holder of the ElyFlow measuring cell contains a nickel electrode. However, this can be replaced by another counter electrode if nickel is not resistant in your electrolyte.
An oxygen gas diffusion electrode is inserted into the working electrode holder of the ElyFlow. This is not supplied with air as the resulting currents are very low.
To coat your sample, impress a correspondingly high current between sample and counter electrode. As soon as hydrogen is produced and diffuses through the sample, the sample becomes a hydrogen electrode on the side facing the gas diffusion electrode.
Hydrogen permeation
Principle for determining hydrogen permeation
Thus, a fuel cell is present which is discharged. The current flowing between the sample and the oxygen electrode is measured amperometrically via a
1 kOhm resistor. The more hydrogen diffuses through the sample, the higher the current.
The AMB automatically records all measurement parameters such as current, cell voltage, potentials against the reference electrodes, temperature, etc. The measurement data is stored on a USB stick and can then be processed further using Excel.
The figure on the right shows the loading potential of the sample as well as the permeation current in nA cm-2 of unalloyed steel of 0.1 mm thickness in 1 M sodium hydroxide solution at room temperature.
First, the sample stand in 1 M sodium hydroxide solution until only a small passivation current is measured.
Then the actual measurement was started with a charging current of 5 mA/cm².
In the figure it can be seen from the negative hydrogen potential that hydrogen is developed immediately at the starting point of the measurement. However, this is only detected after approx. 110 seconds on the other side of the sample. The hydrogen must first diffuse through the sample.
The permeation current increases with increasing measurement time.
After 900 seconds, the charging current on the measuring box was set to
0 mA/cm² again.
The potential rises immediately.
The permeation current slowly subsides as the hydrogen stored in the sheet diffuses out.
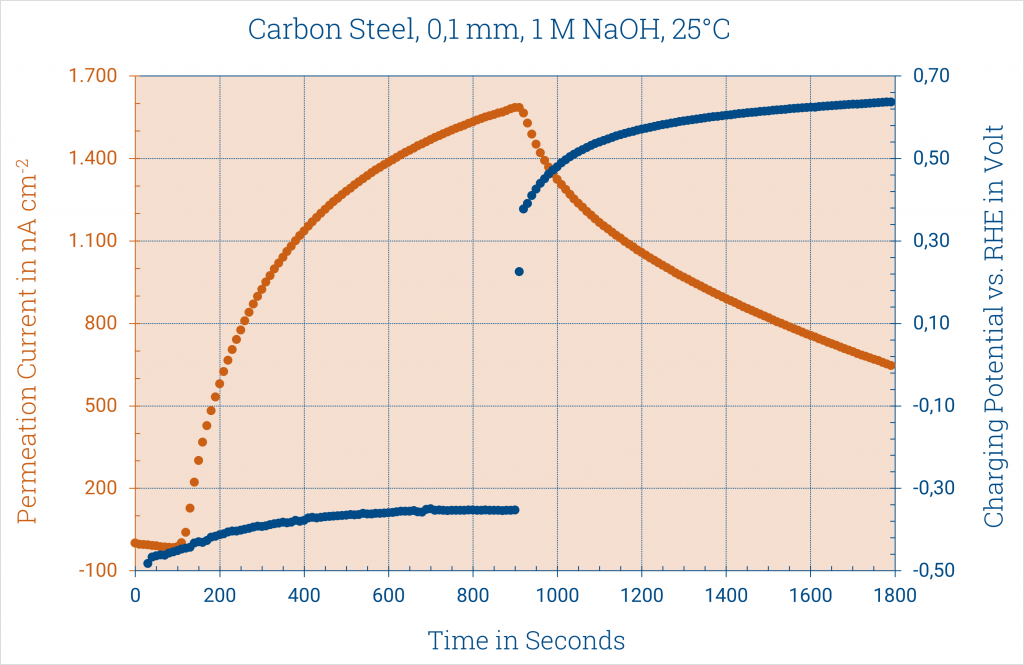
Oxidation current due to the permeation of hydrogen (permeation current) and the charging potential corresponding to the charging current of 5 mA/cm² vs. RHE during hydrogen evolution and the subsequent resting phase at 0 ampere.
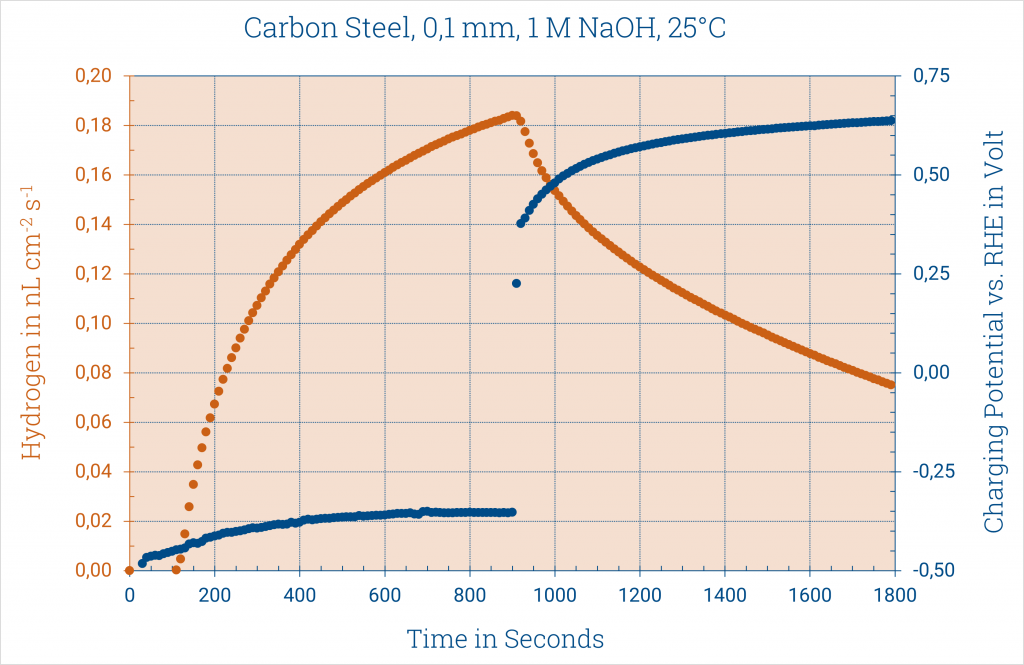
From the current height, Faraday’s law can be used to calculate the hydrogen diffused through the sample.



 Deutsch
Deutsch