Reference electrodes
A reference electrode is a half cell with an electrode potential which establish fast, constantly, repeatable and stable over time. If you state potentials you should always state the reference electrode against which you determined the potential.
If you want to measure conforming to standards you should use the platinum-hydrogen electrode. In doing so, the platinum-hydrogen electrode has to be connected to the negative pole (mass or com entrance). Mass entrances are dedicated a potential of zero. It represents the reference potential for all signal and operating voltages.
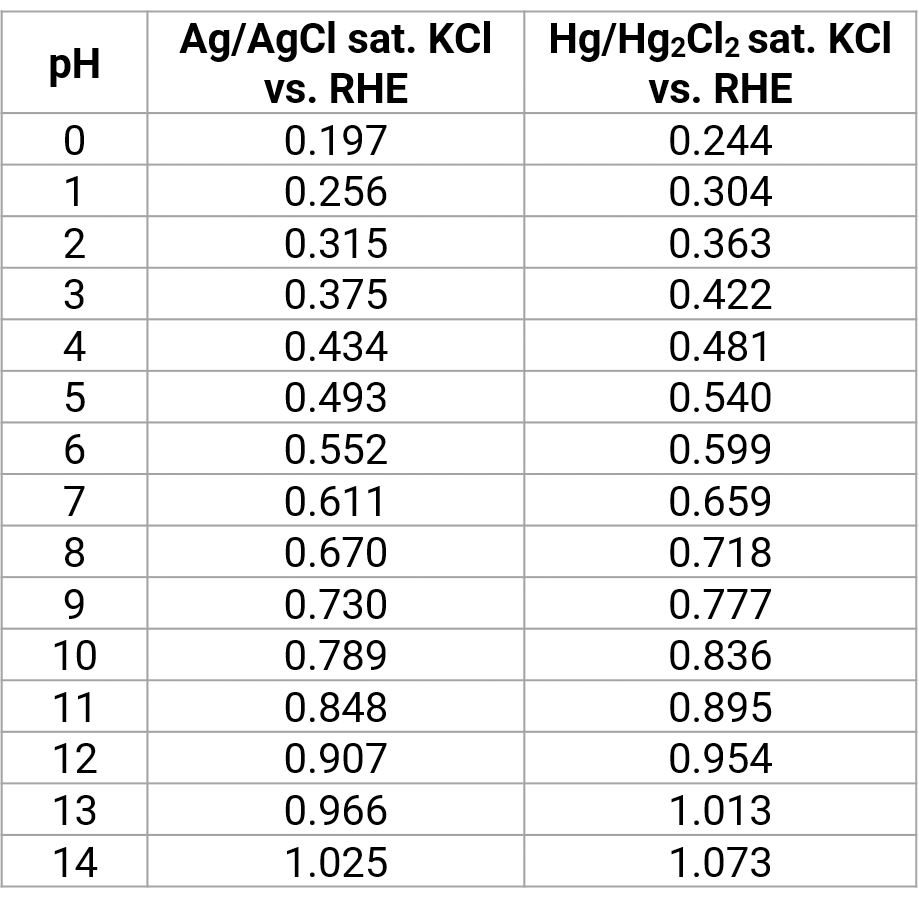
For the platinum-hydrogen reference electrode the hydrogen potential depends on the pH value of the measure solution.
The hydrogen potential depends on the pH value of the measure solution. Here, the potentials of the silver respectively the calomel electrode are plotted against the platinum-hydrogen electrode (RHE – reversible hydrogen electrode placed on mass). With a polarity reversal the algebraic signs reverse suitably.
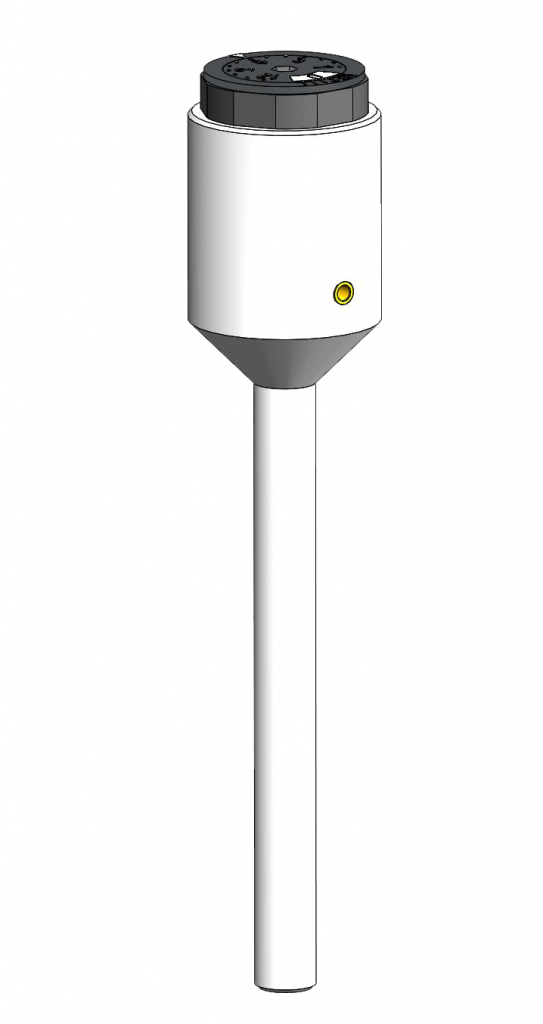
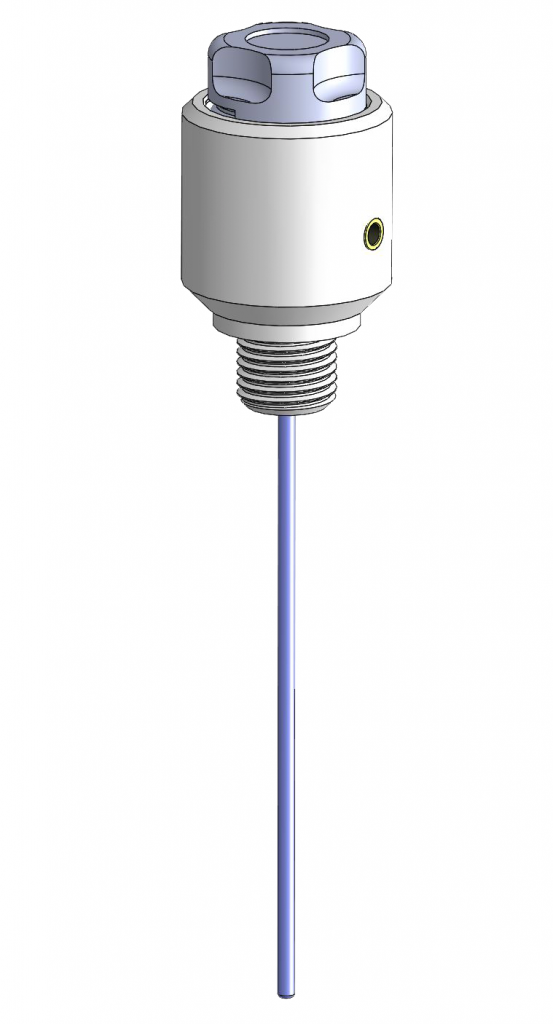
HydroFlex – the platinum-hydrogen reference electrode
The platinum-hydrogen reference electrode is the first choice to measure potentials conforming to standards. Platinum-hydrogen electrodes are the only reference electrodes which measure directly the hydrogen ions activity. If it immerges into the measure solution it is about an indicator electrode.
The hydrogen electrode is crafted completely out of PTFE. It is 120 mm long – as big as a pen – and thus very handy. The shaft has an outside diameter of 8 mm and a length of 80 mm. By activating the hydrogen generation cell (Cartridge) the hydrogen production is started and the interior of the PTFE-tube is charged with hydrogen until it leaks out of the platinum hydrogen electrode.
The voltage is measured at the gold-plated socket in the electrode’s head. Multimeters for measuring voltage should have an input resistance of 5 MOhm and more. Our reference electrode is of low-impedance so that shielded cables are not needed. The HydroFlex can be used up to temperatures of 210°C as long as only the PTFE-shaft is exposed to those temperatures.
The benefits are quite obvious. The electrode provides itself with hydrogen gas by an internal supply which can easily be exchanged. It does not contain interior electrolyte thus there is no ion-discharge and no diffusion potentials. The HydroFlex is low-maintenance because no electrolyte has to be refilled. It does not contain any heavy metals.
The electrode can be used in concentrated fluoride electrolytes and in strongly acidic or strongly alkaline mediums (pH -2 to 16) because it is made out of PTFE.
Before the first use the hydrogen electrode HydroFlex has to be activated thus the hydrogen production in the hydrogen generation cell is started. The arising hydrogen fills the PTFE-tube and leaks out of the platinum-palladium electrode where – according to the ions in the solution – a hydrogen potential sets. The hydrogen potential can be gripped on the gold-plated socket in the electrode’s head. Once activated, the electrode works continuously for 6 months.
Step-by-step manual for the commissioning of the hydrogen electrode
The hydrogen supply has to be activated before the first use. For the functioning of the electrode it is important to stick to the following instructions:
Please do not remove the labelling with the serial number and the sticker with the activation date.
download - Step-by-step manual for the activation of the hydrogen electrode HydroFlexActivation of hydrogen electrode HydroFlex
Storage of the hydrogen electrode HydroFlex
After the measurements you wash up the hydrogen electrode with water. Please, always put the electrodes into a liquid – e.g. measure solution, 1 mol/l hydrochloric acid, 1 mol/l caustic soda – also when you are not measuring. The hydrogen electrodes must not be stored dry in air! We suggest, not to change the duration of the HydroFlex.
Checking the functioning of the HydroFlexReplacement of the cartridge of the HydroFlex
The functioning of the hydrogen electrode is ensured as long as hydrogen is generated (runtime). After 6 months (set and suggested duration) the hydrogen supply has to be replaced. The exceedance of the duration can lead to defects of the hydrogen electrode and should be avoided absolutely. If the replacement of the hydrogen cartridge is not possible the hydrogen electrode has to be taken out of the liquid and be stored dry until a new hydrogen cartridge can be inserted.
You can remove the old hydrogen supply with a SW21-ring-spanner and replace it with a new one. Mind the correct hub of the O-ring when you install the new hydrogen supply. The O-ring must not push itself out. Seal the screw thread with a universal grease (e.g. Korasilon-paste highly viscous). The spent Cartridge has to be disposed of as battery garbage.
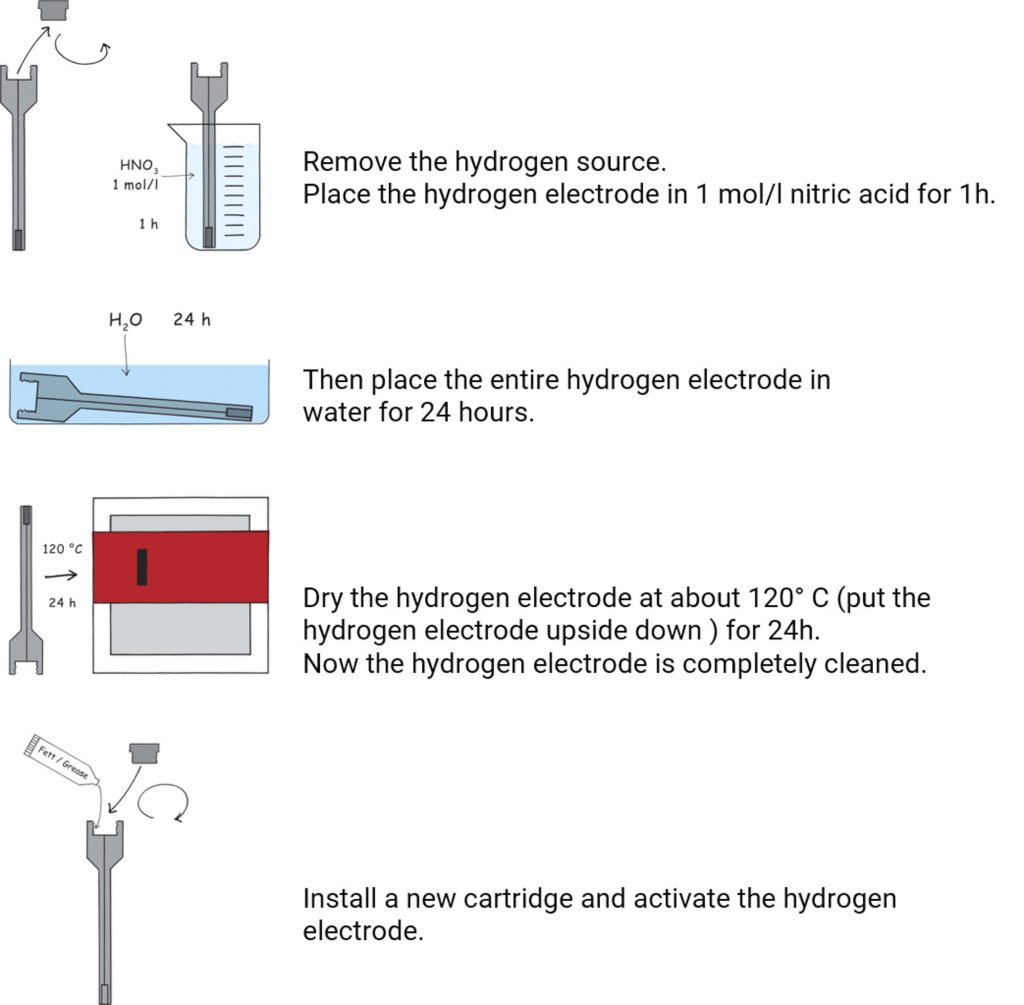
Complete cleaning of the HydroFlex
Sometimes a complete cleaning of the HydroFlex is required – especially when there are visible deposits on the electrode.
download manual - Complete cleaning of the hydrogen electrode HydroFlex
The potential is not displayed correctly.
Cause 1: bad contacting
Check the measuring cables on optical damages such as corrosion, cracks and sessile plugs. Replace the cable. Check the electrode with an external reference electrode, e.g. a calomel or silver silver chloride electrode.
Cause 3: slow ion exchange, e.g. when you switch from concentrated solutions to weak concentrated solutions
Await the setting time, sometimes the balancing of concentrations lasts longer than expected. If necessary, check the potential in another electrolyte such as 1 mol/l hydrochloric acid.
Cause 2: no or too low hydrogen production
Was the hydrogen electrode activated properly?
If not, please activate the hydrogen electrode.
Did you await the activation phase of 24h?
If not, wait 24h before you measure.
Is the duration of the cartridge exceeded?
If yes, please clean the hydrogen electrode as described above and replace the old cartridge with a new one.The new cartridge also has to be activated before you can use it.
Cause 4: air/oxygen gets to the electrode
Avoid that gases such as air or oxygen reach the hydrogen electrode’s shaft. If this happens the hydrogen is displaced or reacts off. No hydrogen potential can be established.
Fluctuating, noisy or oscillating potentials.
Cause 1: hydrogen bubbles of the hydrogen electrode itself
Bubbles are continuously leaking out of the hydrogen electrode. Some bubbles are small, others are bigger. Usually, they do not disturb your measurements.
Is a big bubble forming at the bottom of the hydrogen electrode which sticks to the vessel wall? If possible, place the electrode further away from the vessel wall or hang the hydrogen electrode transversely into the measuring vessel.
Cause 2: induced gases
Change the position of your gas inlet. Induced gas bubbles which are piped by close to the hydrogen electrode can disturb the measurements and lead to fluctuating potentials.
Cause 3: potentiostat or measuring device
Check your measuring device.
If you measure in low conductive electrolytes potentiostats and measuring devices come fast to their limits.
More information about noise in electrochemical measurements can be found here: What-can-cause-my-experiment-to-be-noisy
Deposits (e.g. red or brown) on the electrode.
The hydrogen electrode is contaminated and a mixed potential is set
Check the runtime of the hydrogen supply. If necessary, the hydrogen cartridge must be replaced.
Wipe the electrode with a cloth or clean it in 1 mol/l nitric acid and rinse it with distilled water.
If necessary, you can carry out a complete cleaning.
If that is no successful, the electrode is so badly contaminated that we suggest a new purchase.
Mini hydrogen electrode – Mini-HydroFlex
We developed and downsized our hydrogen electrode for usage within small areas. Composition and functioning comply with our hydrogen electrode HydroFlex – just smaller.
The Mini-HydroFlex is applicable to temperatures of 80°C and works without inner electrolyte. Currently, we provide the Mini-HydroFlex as PEEK-capillary. The capillary has a diameter of 1.6 mm and enables the usage of the Mini-HydroFlex in small openings.
The capillary’s standard length is 53,5 mm, other lengths are available for an additional charge. Thus the Mini-HydroFlex can be individualised in accordance with your wishes.
download manual mini-HydroFlexBefore you measure, you must insert the supplied hydrogen cell (button cell) into the electrode head and place the mini hydrogen electrode in water for 24 hours preferably.
Please do not remove the labelling with the serial number and the sticker with the activation date.
Step-by-step manual for the commissioning of the mini hydrogen electrode mini HydroFlex
Storage of the mini hydrogen electrode Mini-HydroFlex
After your measurements rinse the electrode thorough with water. Even when you are not measuring, please always place the mini hydrogen electrode in a liquid, e.g. measuring solution, 1 mol/l hydrochloric acid or 1 mol/l caustic soda. Hydrogen electrodes must not be stored dry in air!
Replacement of the hydrogen cell
After reaching the runtime of 12 months the hydrogen button cell has to be replaced. The exceedance of the duration can lead to defects of the hydrogen electrode and should be avoided absolutely. If the replacement of the hydrogen cell not possible the hydrogen electrode has to be taken out of the liquid and be stored dry until a new hydrogen cell can be inserted.
download - Replace of the hydrogen cell in Mini-HydroFlex
The potential is not displayed correctly.
Cause 1: bad contacting
Check the measuring cables on optical damages such as corrosion, cracks and sessile plugs. Replace the cable. Check the electrode with an external reference electrode, e.g. a calomel or silver silver chloride electrode.
Cause 3: slow ion exchange, e.g. when you switch from concentrated solutions to weak concentrated solutions
Await the setting time, sometimes the balancing of concentrations lasts longer than expected. If necessary, check the potential in another electrolyte such as 1 mol/l hydrochloric acid.
Cause 2: no or too low hydrogen production
Did you place the new Mini-HydroFlex in the measuring solution for 24 hours?
If not, wait 24 hours before you measure.
Is the duration of the hydrogen source exceeded?
If yes, please replace the hydrogen cell.
Cause 4: air/oxygen gets to the electrode
Avoid that gases such as air or oxygen reach the hydrogen electrode’s shaft. If this happens the hydrogen is displaced or reacts off. No hydrogen potential can be set.
Fluctuating, noisy or oscillating potentials.
Cause 1: hydrogen bubbles of the hydrogen electrode itself
Bubbles are continuously leaking out of the hydrogen electrode. Some bubbles are small, others are bigger. Usually, they do not disturb your measurements.
Is a big bubble forming at the bottom of the hydrogen electrode which sticks to the vessel wall? If possible, place the electrode further away from the vessel wall or hang the hydrogen electrode transversely into the measuring vessel.
Cause 2: induced gases
Change the position of your gas inlet. Induced gas bubbles which are piped by close to the hydrogen electrode can disturb the measurements and lead to fluctuating potentials.
Cause 3: potentiostat or measuring device
Check your measuring device.
If you measure in low conductive electrolytes potentiostats and measuring devices come fast to their limits.
More information about noise in electrochemical measurements can be found here: What-can-cause-my-experiment-to-be-noisy
Deposits (e.g. red or brown) on the electrode.
The hydrogen electrode is contaminated and a mixed potential is set
Check the running time of the hydrogen source. If necessary, the hydrogen cell must be replaced.
Some deposits can be wiped off with a cloth.
With 1000 grit sandpaper, you can carefully sand the bottom of the electrode.
More stubborn impurities dissolve in 1 mol/l nitric acid. The electrode must then be rinsed thoroughly in distilled water.
If the cleaning steps are unsuccessful, the electrode is unfortunately so contaminated that we recommend buying a new one.












 Deutsch
Deutsch