Principles of the pH-value measurement with hydrogen electrodes
Hydrogen potential and pH-value
The hydrogen potential directly depends on the activity or rather the concentration of the hydronium ions and thereby on the pH-value of the solution. The pH-value itself is defined as the negative decadic logarithm of the hydrogen ions activity. The activity is the product of concentration and activity coefficient. Latter can only be defined approximately in diluted solutions. Thus, the measurement of the pH-value according to its definition can only be made approximately.
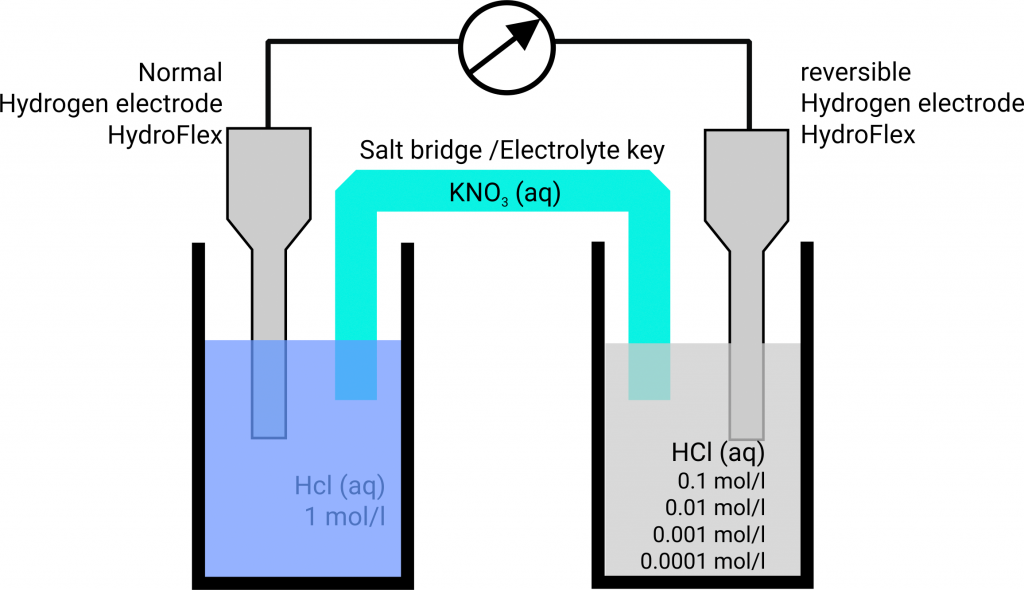
If two platinum hydrogen electrodes, of which one is immersed in an acid of protons concentration 1 mol/l, pH = 0, (normal hydrogen electrode, NHE) and the second in a solution of unknown protons concentration (reversible hydrogen electrode, RHE), are contacted via a salt bridge you get a concentration chain or rather a concentration cell.
As soon as two half cells are contacted and the circuit is closed the potential of the spontaneous happening reaction sets. Due to the higher proton concentration in the left half-cell the hydrogen ions are reduced to hydrogen. Thereby, electrons are removed from the electrode thus it has a more positive potential than the electrode in the right half-cell. So, the reduction takes place in the left half-cell. That cell is equivalent to the cathode and is the positive pole in the galvanic cell. In the right half-cell hydrogen is oxidized to hydrogen ions. The electrode has a more negative potential than the left electrode (negative pole). In the right half-cell the oxidation takes place so that the right half-cell is equivalent to the anode. The freely flow direction of the electrons is from the negative pole to the positive pole, here (galvanic cell) from the anode to the cathode. Electrons flow from the right to the left, thus from the diluted to the concentrated cell.
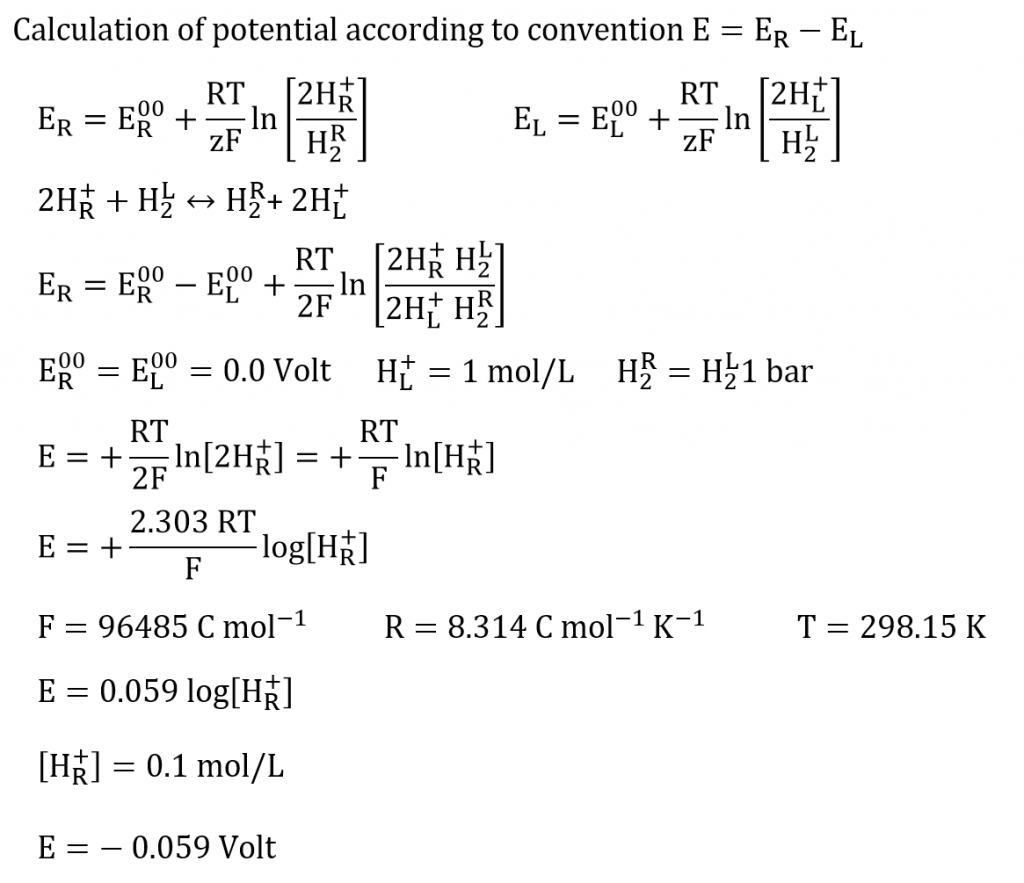
The potential is calculated by E = E (right) – E (left). The standard hydrogen electrode has to be the left cell according to the IUPAC.
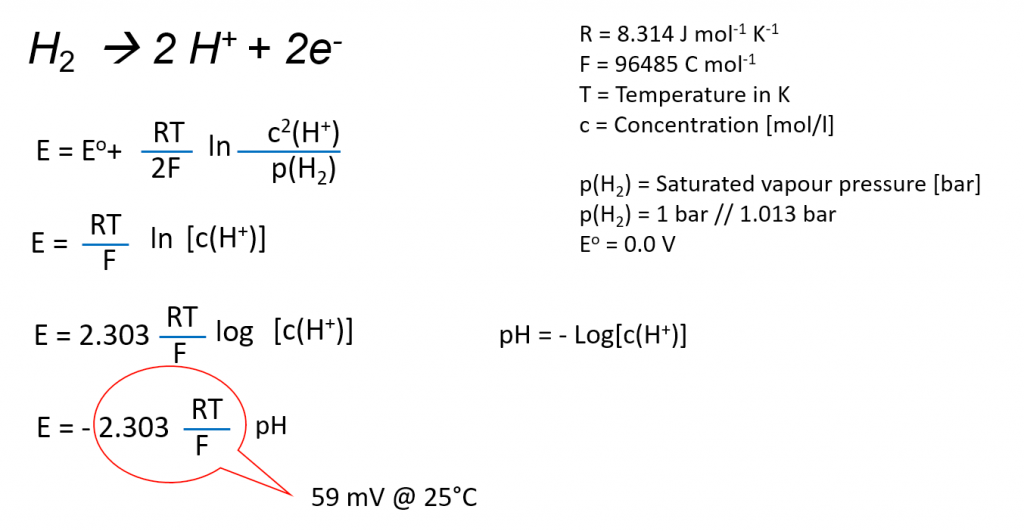
The potential changes technically with a positive sign depending on the proton concentration.
BUT: we have a logarithmic function of the protons function. For concentration < 1 mol/l we get negative potentials, here there are -0.059 V for a concentration of 0.1 mol/l. if you convert the protons concentration into the pH-value the calculation of the potentials is easier.
E = 0.059 pH (25°C)
Since E < 0 Volt, the reaction runs from the right to the left as we are expecting it according to the different concentrations. To measure negative potentials in such a concentration chain you have to connect the standard hydrogen electrode to the negative pole (COM-port).
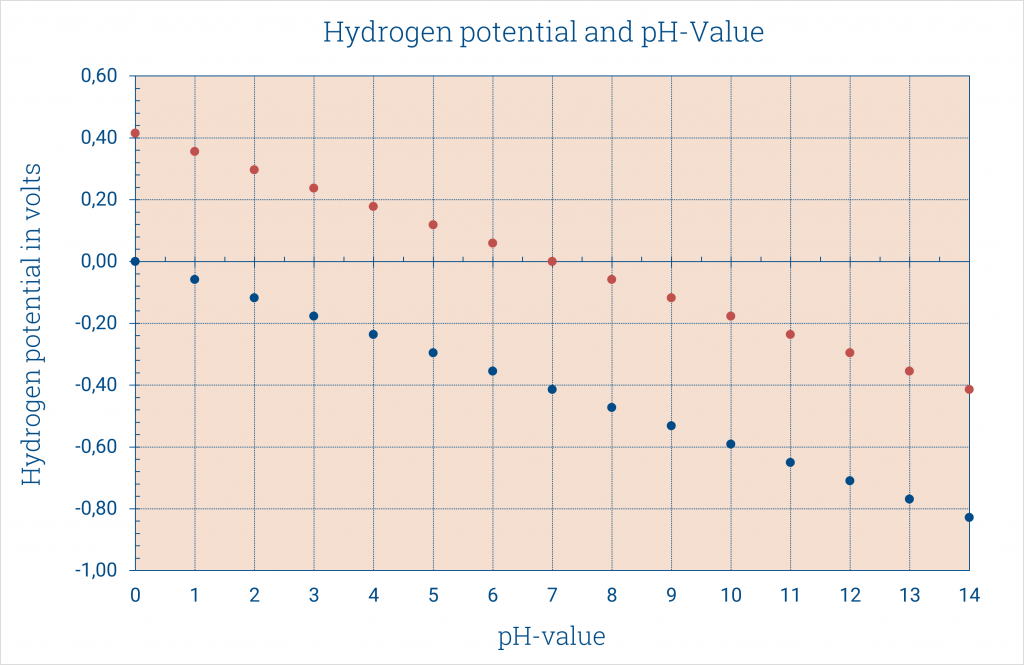
The platinum hydrogen pH-electrode pHydrunio is such a measuring chain. The outer hydrogen electrode measures the hydrogen potential in the measuring solution. Against a normal hydrogen electrode (per definition: reference potential of 0 mV) the blue straight in the diagram arises, according to the calculation E = -0.059 pH (25°C).
But, the inner reference electrode of our pH-electrode is placed in buffer pH 7. That has to be considered in the calculation of the potential.
Hence, an offset of 0.414 V arises at 25°C and the measuring voltages are equivalent to the orange straight.



 Deutsch
Deutsch