pH-electrode pHydrunio
Gaskatel’s phydrunio pH electrode is the world’s first pH electrode combining
two hydrogen electrodes into a combination electrode.
pH-electrodes
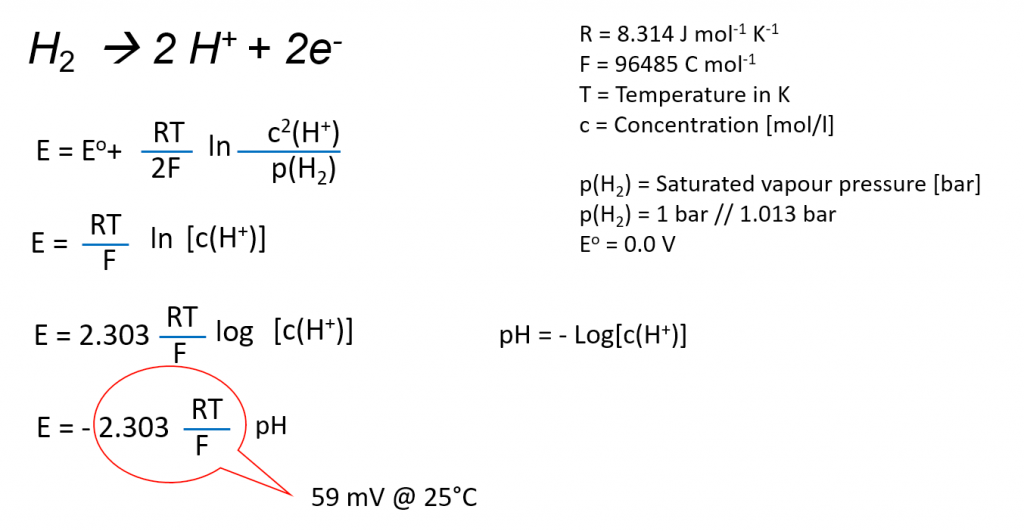
The platinum hydrogen electrode is the only electrode whose potential is determined directly by the hydrogen ions activity. The pH-value and the hydrogen potential are directly connected with each other. The potential, depending on the pH-value, can be calculated by means of the Nernst equation.
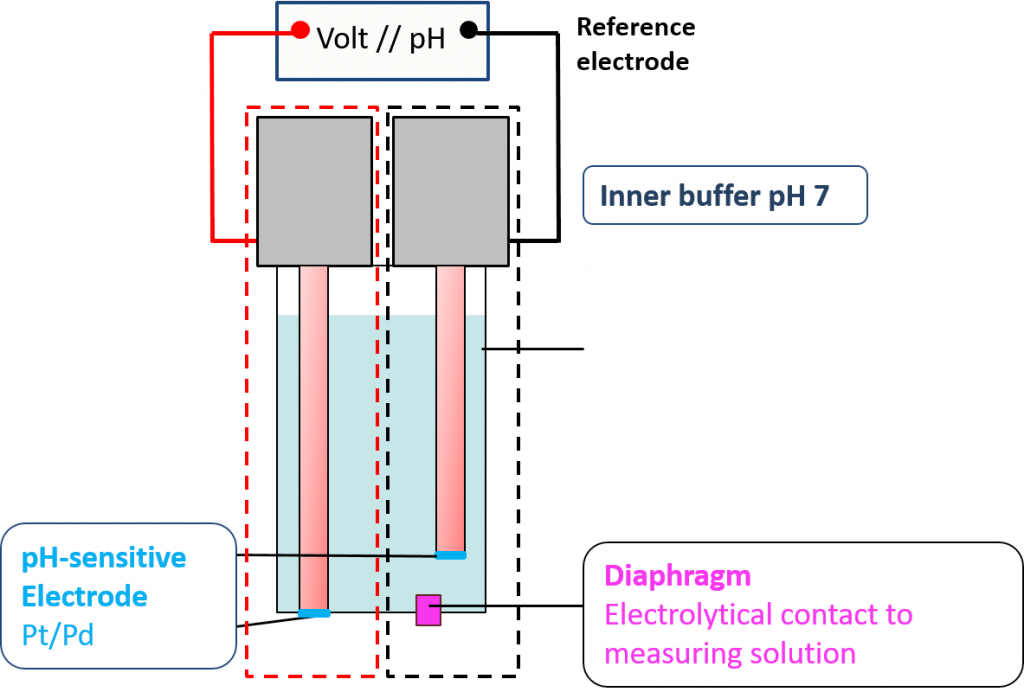
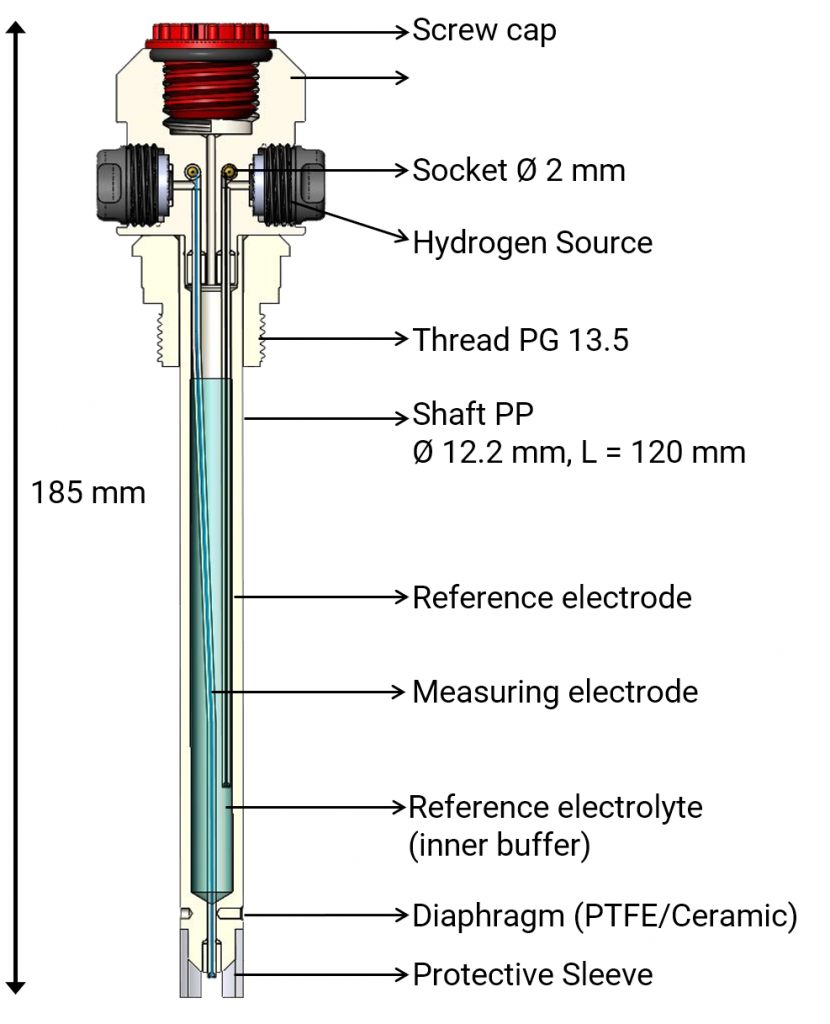
So, it comes naturally to mind to combine two platinum hydrogen reference electrodes to a combination electrode to create a new platinum hydrogen pH-electrode. That was enabled by the miniaturization of the hydrogen electrode HydroFlex. Two platinum hydrogen electrodes are combined to a pH-electrode. The interior hydrogen electrode is the reference electrode. It must immerse in a buffer of a known pH-value. The ionic connection to the measuring solution is made by a diaphragm. The outer platinum hydrogen electrode is the actual measuring electrode (outer electrode). The voltage is tapped via a voltmeter between the two platinum hydrogen electrodes.
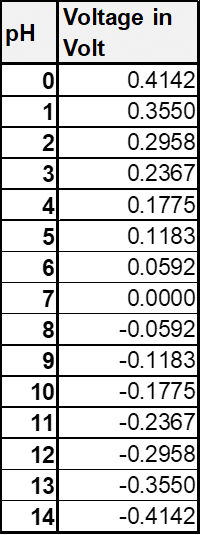
The measuring voltage and the pH-value are related to each other as shown in the following:
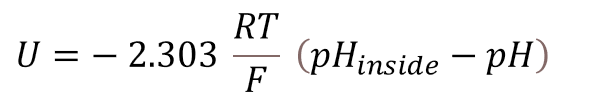
The measuring voltage can be converted into the pH-value by adjusting the equation.
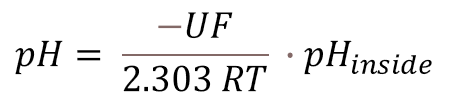
U = Measured voltage in Volt
F = Faraday constant 96485 C mol1
R = Universal gas constant 8,314 J mol-1 K1
T = Temperature in Kelvin K
pHinside = 7
Simplified the equation for the converting of the measured voltage U into the pH-value is for 25°C:
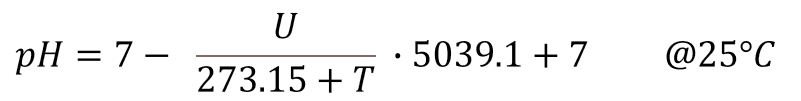
pHydrunio – the platinum hydrogen pH-electrode
pHydrunio (Patent DE 10 2011 113941) is worldwide the first platinum hydrogen pH-electrode for sale and the logical further development of our mini platinum hydrogen electrode Mini-HydroFlex. It combines a reversible platinum hydrogen electrode as the measuring electrode with a platinum hydrogen reference electrode in an interior electrolyte (pH 7) as the reference electrode. It is delivered activated and supplied with hydrogen gas by an internal supply for 12 months. Then, the hydrogen cells can be replaced. The hydrogen inside the shaft ensures the controlled electrolyte output out of the diaphragm. However, if measuring solution enters the electrode the interior electrolyte can be replaced. pHydrunio is made of polypropylene and thereby indestructible.
Thus, it can be used in highly alkaline or acid electrolytes as well as in concentrated fluoride media.
The shaft is 120 mm long and the diameter is 12.2 mm. A PG 13.5 thread enables the installation in appropriate armatures. The measuring electrode is protected from mechanical damages by a protective sleeve. pHydrunio can be connected to any pH meter with analogue input (BNC or open wire ends).
In contrast to the usual glass electrodes, our hydrogen pH electrode has a low resistance.
The platinum hydrogen pH-electrode pHydrunio is delivered pre-assembled which means the hydrogen cells are already installed and activated. On the electrode’s head you can find a label which states the month of activation. Do not remove that label! pHydrunio is already filled with an interior electrolyte.
Before you start your measurements please place the hydrogen pH-electrode in distilled water for 24 hours.
Do not remove neither the label with the month of activation nor the label with the serial number.
Storage of the platinum hydrogen pH-electrode pHydrunio
After the measurements, rinse the hydrogen pH electrode thoroughly with water. Please place the pH electrode in a vessel with distilled water. Under no circumstances it should be stored in the transport cap. The hydrogen would squeeze slowly the small volume of liquid out of the cap. The hydrogen pH electrode should not be stored dry in air as long as the hydrogen cells are generating hydrogen. A mixed potential forms which can lead to incorrect results. Pay attention to the runtime of the hydrogen cells.
Replacement of the hydrogen cells
The functioning of the hydrogen pH-electrode is ensured as long as hydrogen is generated (runtime). The run time is 12 months. As soon as the running time of 12 months is reached, the hydrogen cells must be replaced immediately. Otherwise, a negative pressure will build up in the capillaries so that liquid can get into the capillaries.
Calibration of pHydrunio
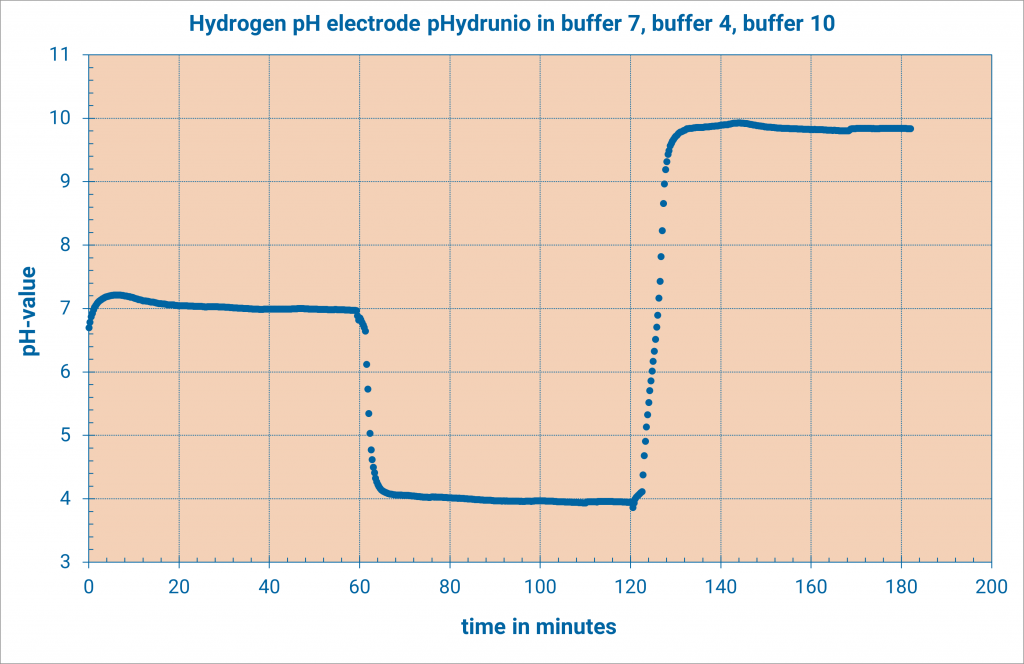
First of all, the pH-electrode is placed in calibration buffer pH 7. Thereby the deviation from the zero point is determined. According to the condition of the electrode it can last several minutes. Note the measured voltage and the temperature of the buffer as soon as the voltage has become constant.
Then, rinse the electrode and the temperature sensor with water before you place both in calibration buffer pH 4 or pH 10. Note the measured voltage and the temperature of the buffer as soon as the voltage has become constant. Waiting times of several minutes are possible.
Inspection of the functioning of pHydrunio
You can check both electrodes in the hydrogen pH-electrode separated from each other against a so-called master electrode, e.g. a calomel electrode. The master electrode should always be placed in appropriate potassium chloride solution and not be used for other purposes.
Pay attention to the fill level in the calomel electrode itself. It must always be above the fill level in the measuring vessel. Open the filling plug of your calomel electrode to prevent the measuring fluid from entering the calomel electrode.
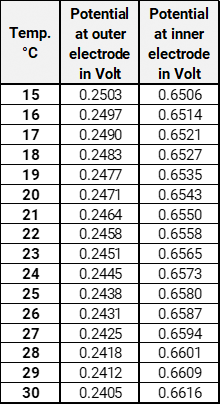
To check the hydrogen pH-electrode pHydrunio, place pHydrunio and the master electrode in buffer pH 0. Connect the calomel electrode to the positive pole. To contact the pH-electrode you need an appropriate adapter BNC on 4 mm laboratory socket. Now, connect the red socket of the adapter with the negative pole (COM-port) of your voltmeter. You are now checking the outer measuring electrode of the hydrogen pH-electrode. After an adjustment phase, which can last several minutes, you read off the potential. Now, connect the black socket of the adapter with the negative pole (COM-port) of your voltmeter. You are checking the inner reference electrode of your pH-electrode. After an adjustment phase, which can last several minutes, you read off the potential.
The following table shows the to expecting potentials. It is assumed that the pH-values stay constant in these temperature ranges. Diffusion voltages are not considered.
Download Potentials in buffer pH 0Replacement of the internal buffer
The internal buffer (reference electrolyte) can be replaced. Therefore, the sealing cap at the electrode’s head must be unscrewed. You can pull the internal buffer out with the provided syringe and cannula.
Rinse out the electrode with distilled or deionized water. Fill in the new internal buffer directly out of the vial. Screw down the pHydrunio carefully. Place pHydrunio in distilled water for 24 hours.
pH-value (potential) is not displayed correctly
Cause 1: buffer solution
Please measure in fresh buffer solution.
If buffer solutions are exposed to air for a longer time, the pH value can shift.
This occurs mainly with alkaline buffer solutions.
Cause 4: bad contacting
Check the measuring cables on optical damages such as corrosion, cracks and sessile plugs. Replace the cables. Check both electrodes with an external reference electrode, e.g. a HydroFlex, calomel or silver silver chloride electrode.
Cause 2: slow ion exchange, e.g. when you switch from concentrated solutions to weak concentrated solutions
Await the setting time, sometimes the balancing of concentrations lasts longer than expected. If necessary, check the potential in another electrolyte, e.g. in calibration buffers.
Cause 5: air/oxygen gets o the hydrogen electrode
Avoid that gases such as air or oxygen reach the platinum hydrogen electrode’s shaft. If this happens the hydrogen is displaced or reacts off. No hydrogen potential can be set.
Cause 3: internal buffer contaminated
It can happen that measuring solution enters the internal buffer and contaminates the internal buffer.
In that case, the internal buffer has to be replaced
Cause 6: no or to low hydrogen production
Is the runtime of the hydrogen cells exceeded?
If yes, please replace the hydrogen cells.
Fluctuating, noisy or oscillating pH values (potentials)
Cause 1: hydrogen bubbles of the hydrogen electrode itself
Bubbles are continuously leaking out of the platinum hydrogen electrode. Some bubbles are small, others are bigger. Usually, they do not disturb your measurements.
Is a big bubble forming at the bottom of the hydrogen electrode which sticks to the vessel wall?
If possible, place the electrode further away from the vessel wall or hang the hydrogen electrode transversely into the measuring vessel.
Cause 2: induced gases
Change the position of your gas inlet. Induced gas bubbles which are piped by close to the hydrogen electrode can disturb the measurements and lead to fluctuating potentials.






 Deutsch
Deutsch