Reference electrodes
How do electrochemical potentials arise?
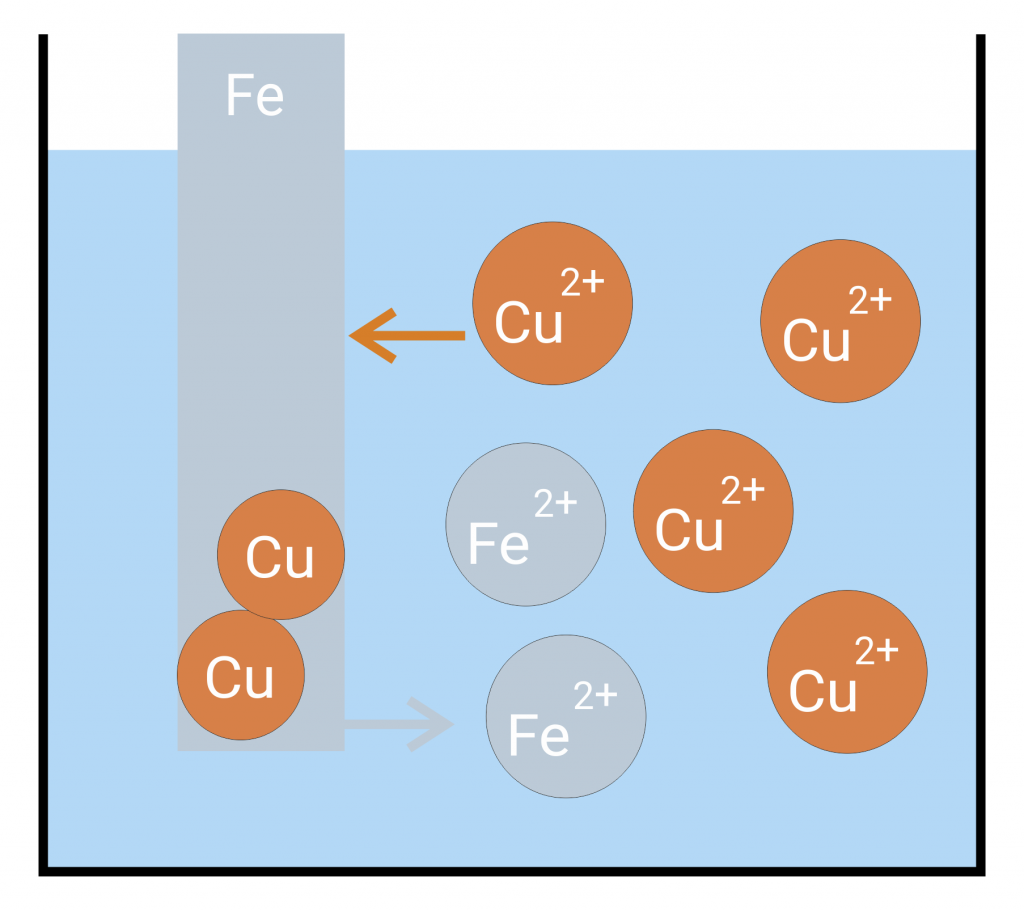
Voltages and potentials arise between an electron conductor (electrode) and an ion conductor (electrolyte) as soon as they are brought into contact with each other. Charges are exchanged until the system is balanced. Example: If a sheet iron is immersed in copper saline a copper precipitation is formed on the iron. If you reverse the experiment (immerse a sheet copper in ferrous saline) nothing happens.
How do these two metals differ from each other?
An equilibrium between the metal and the liquid arises when they are brought into contact. Metal ions dissolve out of the lattice of the metal which is immersed in a liquid of its own or other metal ions. That is known as solution pressure which is yielded by the difference of the lattice energy and the hydration energy. In return, metal ions are pressed back into the metal lattice by the separation pressure.
In the example above, that means that iron ions go into the solution while the copper ions present in the solution absorb the remaining electrons in the sheet iron and separate as copper on the sheet iron. If you reverse the experiment, neither copper goes into the solution nor iron ions are separated. Thus, the solution pressure of iron is higher than the solution pressure of copper.
If the solution pressure dominates an excess of electrons arises at the metal rod. If the separation pressure dominates a shortage of electrons arises at the metal rod. A dynamic equilibrium is formed. The separation of the metal ions is thereby supported by the electric attraction of the negatively charged metal. The solution pressure, the separation pressure and the electric pressure lead to a state of minimal energy in which electric work is done. The electric work can be seen in the form of voltage at the metal rod.
That absolute equilibrium voltage as such cannot be determined experimentally since only voltage differences are experimentally accessible. Therefore, a second electrode is needed which is called comparative electrode, reference electrode, indifferent electrode or indicator electrode. Its equilibrium voltage cannot be measured separately, too. To compare the voltages of different metals in their solutions they have to be measured against the same reference electrode. Therefore, you should use a reference electrode whose equilibrium voltage sets fast and reproducibly.
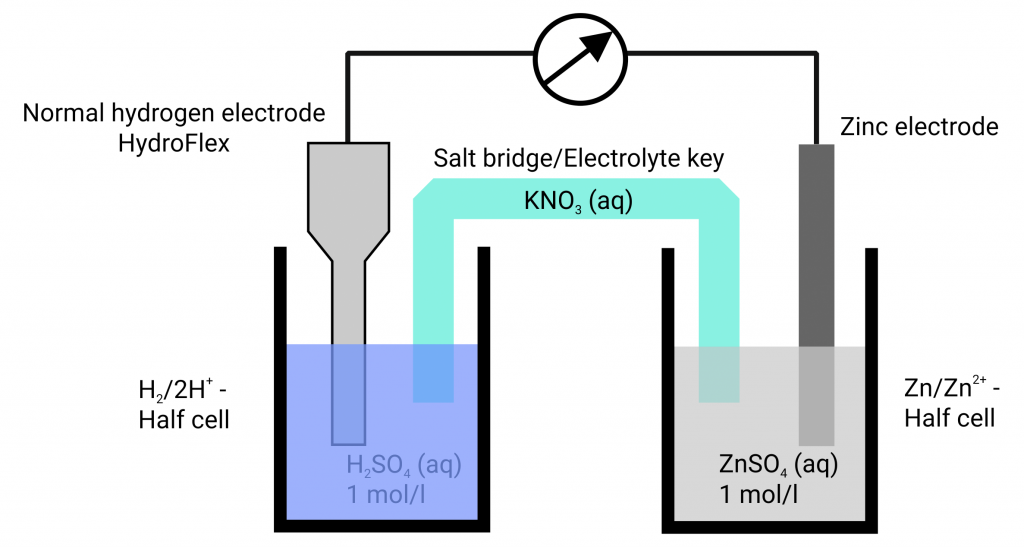
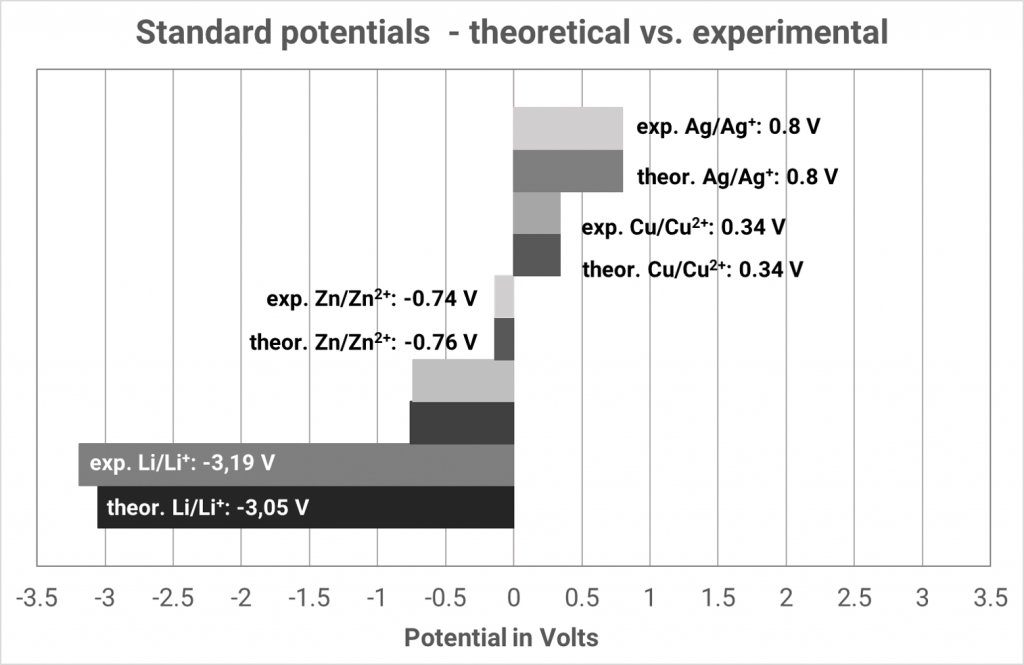
In the past, a platinum plated platinum electrode washed round with hydrogen gas in hydrochloric acid has established itself. The equilibrium voltage at standard conditions (hydronium ion activity of the hydrochloric acid 1 mol/l; hydrogen gas 1.013 bar) has been defined as 0.000 V at all temperatures. Only then, the hydrogen electrode is designated as a standard hydrogen electrode. If the voltage differences of various metals in their metal salt solutions are measured against the standard hydrogen electrode, different voltages arise which can be higher or lower than 0.000 V.
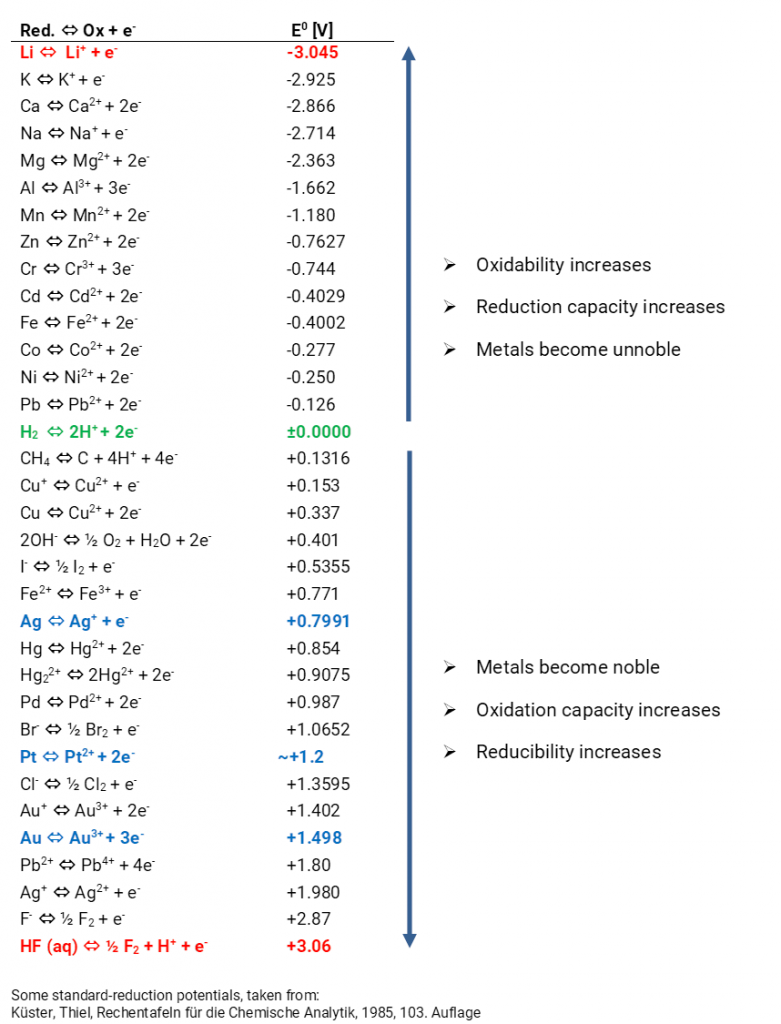
The expected equilibrium potentials of different systems at standard conditions (25°C, metal ion activity 1 mol/l) are collated in the electrochemical series. Metals with an equilibrium potential lower than 0 V are designated as ignoble. If the equilibrium potentials are higher than 0 V the metals are designated as noble.
In accordance with the electrochemical series you can also state equilibrium potentials of non-metals and their ions as well as ionic compounds and their charge reversals. If a reactant is oxidised a reduction product forms. Reversed, an oxidation product forms if a reactant is reduced. So, there are always redox couples. The equilibrium potentials are a measure for the oxidation or reduction capacity. The more negative the potential is all the easier the metals emit electrons. They are oxidised and function as a reducing agent. The higher the potential is all the more complicated it is to oxidise electrons. Instead, they are easier to reduce and thus function as an oxidant. The expected voltages for various combinations of half cells can be calculated with the standard potentials. Predictions about possible or impossible chemical reactions in aqueous solutions can be made. The convention E = E (right) – E (left) is authoritative for the calculation of the expected voltage.
Activity and concentration – both terms are used in the electro chemistry. But what is the difference?
The concentration of substance c indicates the amount of substance n of a dissolved species in a defined volume and thus the unit is mol/l. Furthermore, in the chemistry the designation molarity M is usual for the concentration of substance in mol/l. The concentration of substance is dependent on the temperature because the volume is temperature-dependent. You can also relate the concentration of substance of a dissolved species to the weight of the solvents in kg. Then, you get the molality b in mol/kg. The molality is temperature-independent. The molality is especially found in older tables. Moreover, one can find the term of the activity of a species. The activity of a substance is a thermodynamic value, a kind of a corrective concentration.
In a solution the charged ions are surrounded by solvation shells (hydrate shells in water as solvent) and thus sealed off each other. The ions are less effective. The activity corresponds to the remaining effective concentration which, in general, is lower than the concentration because of the interactions between the ions in the solution. Only in very diluted solutions the activity and the concentration are almost equal. In high concentrated solutions the activities can reach higher values than the concentration because there are not enough solvate molecules to solvate all the ions completely. The deviation between the activity and the concentration is shown in a correction factor, the so-called activity coefficient y, γ or f (depending on the kind in which the concentration of substance is stated).
Universal definition of the activity (does not correspond to the physicochemical definition) according to Jander, Jahr; Maßanalyse; 15. Auflage, 1989, de Gruyter:
a = y c c = concentration of substance in mol/l (molarity)
a = γ c c = concentration of substance in mol/kg (molality)
a = f c c= content of the dissolved substance as mole fraction
Potential – voltage – electromotive power
All three terms stand for a voltage difference between two systems. If the reference point for the measurement is known you speak of a potential otherwise you speak of voltage.
Electromotive power means the potential difference of a cell which is measured if the cell works reversible and no current flows.
As per the convention of the IUPAC1 a standard potential (or standard voltage) E is calculated by the equation
E = E(right) – E(left) (standard electrode potential of the right cell minus standard electrode potential of the left cell).
1: Cohen et al: Quantities, units and symbols in physical chemistry, IUPAC Green Book, 3rd edn., 2nd printing, IUPAC & RSC Publishing, Cambridge, 2008, S. 71
The IUPAC² also determines: “The standard potential of an electrochemical reaction, abbreviated as standard potential, is defined as the standard potential of a hypothetical cell, in which the electrode (half-cell) at the left of the cell diagram is the standard hydrogen electrode (SHE) and the electrode at the right is the electrode in question.“
2: Cohen et al: Quantities, units and symbols in physical chemistry, IUPAC Green Book, 3rd edn., 2nd printing, IUPAC & RSC Publishing, Cambridge, 2008, S. 74
If you always consider the standard hydrogen electrode as the “left” cell, the standard potentials of the electrochemical series automatically arise, indicated as standard reduction potential. In old tables one often finds standard oxidation potentials, then you have to reverse the algebraic signs. You can identify whether in a table the oxidation or reduction potentials are indicated when you observe the algebraic signs of the reaction Cu/Cu2+ since then the standard potential is +0.34 V.
In doing so, the question which electrode has to be connected to which input of the measurement instrument arises because according to the connection of the electrodes the algebraic sign of the measured potential changes. The values of the electrochemical series are the result of the measurements if you connect the standard hydrogen electrode to the minus input (COM). If you measure against another reference electrode you connect it to the minus input (COM). Thus, the measurement electrode is always connected to the positive pole: E = E(measurement electrode) – E(reference electrode).
The algebraic sign of the measured potentials shows which electrode is more positive, in which direction the current flows and which of the reaction runs spontaneously since the potential is directly related to the equilibrium constant of the reactants. E > 0 for E(right) > E(left) means that the right electrode is positive charged towards the left electrode.
There is a shortage of electrons on the right electrode caused by the reduction of particles. That electrode is also called cathode where the reduction happens. Therefore, the electrode flow from the left to the right. Thus, the electric current, unfortunately defined as a flow of positive particles, flows in opposite direction. On the left electrode there is an excess of electrons since there particles are oxidised. That electrode is called anode where the oxidation takes places. By trend, the reaction runs spontaneously from the left (oxidation) to the right (reduction). E < 0 for E(right) < E(left) by implication means that the reaction runs spontaneously from the right to the left.
Measuring potentials – also in school
The measuring of potentials happens almost currentlessly. The electrode of which you want to determine the potential is connected, in an appropriate solution, to a reference electrode via a measuring instrument. According to the measuring conditions, special requirements are made to the test cell. The used measuring instrument used can also have an impact on the measurements. The input resistance of the measuring instruments used can, according to the internal resistance of the reference electrode, affect or even falsify the potential measurement. With commercial reference electrode the potential measurement is only currentless if the measuring instruments are of high-resistance. Otherwise, electrochemical conversions can happen. The hydrogen reference electrode HydroFlex has a low internal resistance so that one can use handheld multimeters for the potential measurement – as long as the to study system is of low-resistance.
In our opinion, HydroFlex should be part of every school’s equipment since the standard potentials can be measured conforming to standards at simple systems. You can use HydroFlex in a beaker with sulphuric acid or hydrochloric acid (concentration of 1 mol/l) and get a standard hydrogen electrode instantly. Connect that vessel with the to measure system via a salt bridge (easiest case: a filter paper soaked in KNO3). Your students can directly read off the standard potentials at the multimeter and compare them with the tables.
LEYBOLD offers our hydrogen electrode in experiment „Standardpotenziale von Metallen“ (C4.4.3.2B).
D. Böhm, T. Kurzenknabe, M. Schwab, E. Geidel: Experimente zur Bestimmung von Standardpotenzialen im Chemieunterricht, Praxis der Naturwissenschaften, Chemie in der Schule, Aktuelle Entwicklungen in der Elektrochemie, Heft 8, 64. Jahrgang, Dezember 2015, Friedrich Verlag.
Risk assessment: Messung von Standardpotenzialen, Redoxpotenzial und Konzentration You can find more information or suggestions for the use of HydroFlex in school here: www.fachreferent-chemie.de.
HydroFlex as a practical hydrogen electrode in school has been presented on the Bundeskongress 2014 of the MNU in Kassel. You can find the script including the lecture script including the lecture here.
Reference electrodes with salt bridge (reference electrodes of second kind)
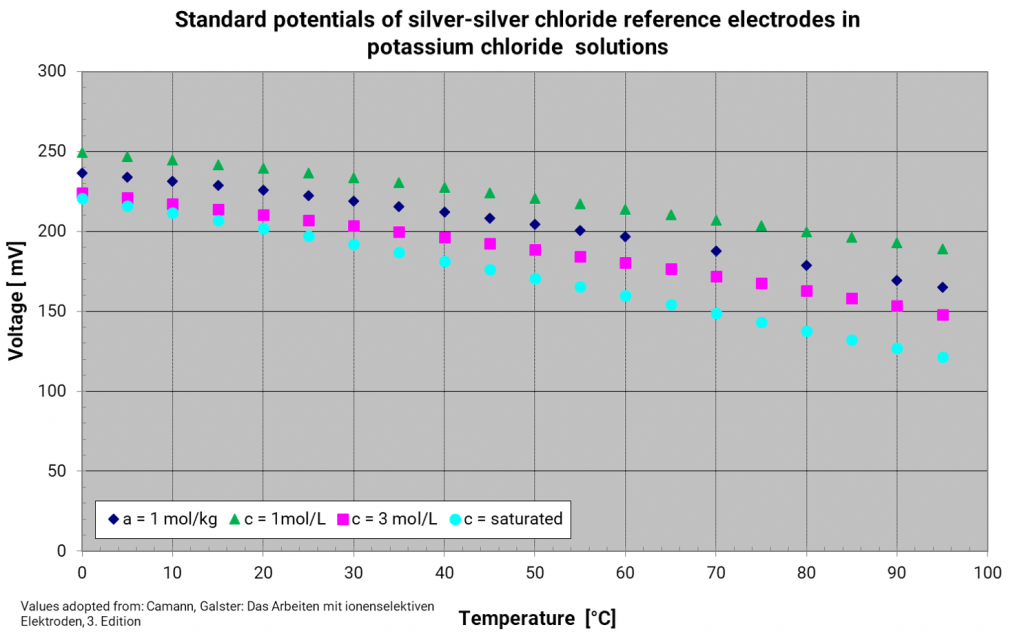
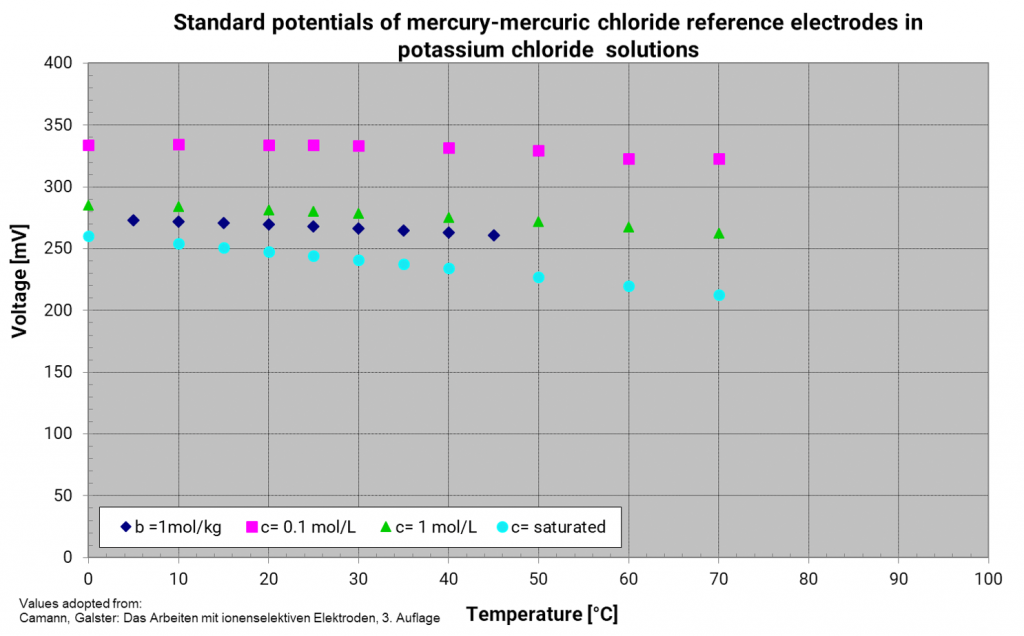
In former years, other systems besides the reversible hydrogen electrode have established as reference electrodes, but they are only usable with an interior electrolyte and an appropriate electrolyte bridge. They are reference electrodes of the second kind. These reference electrodes should be chosen according to the measuring solution to avoid contamination and unneeded diffusion voltages. Moreover, the diaphragm has not to be so fine-pored which reduces the transition resistance between the measuring solution and the reference electrode. For aqueous and acidic solutions, the following references have established:
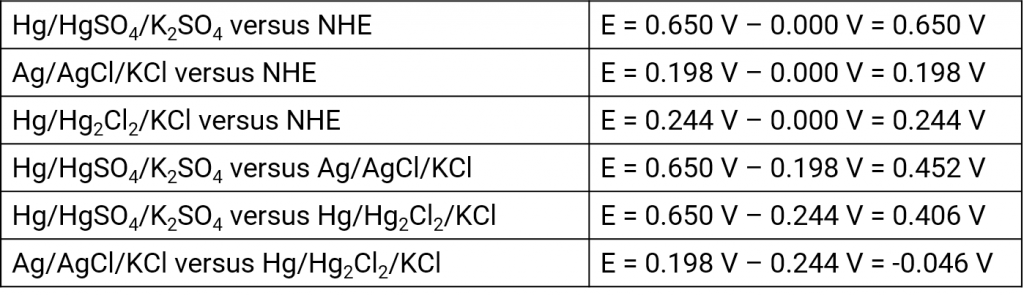
Silver silver chloride electrode
Mercury mercury chloride electrode (calomel electrode)
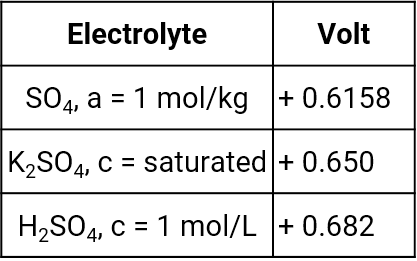
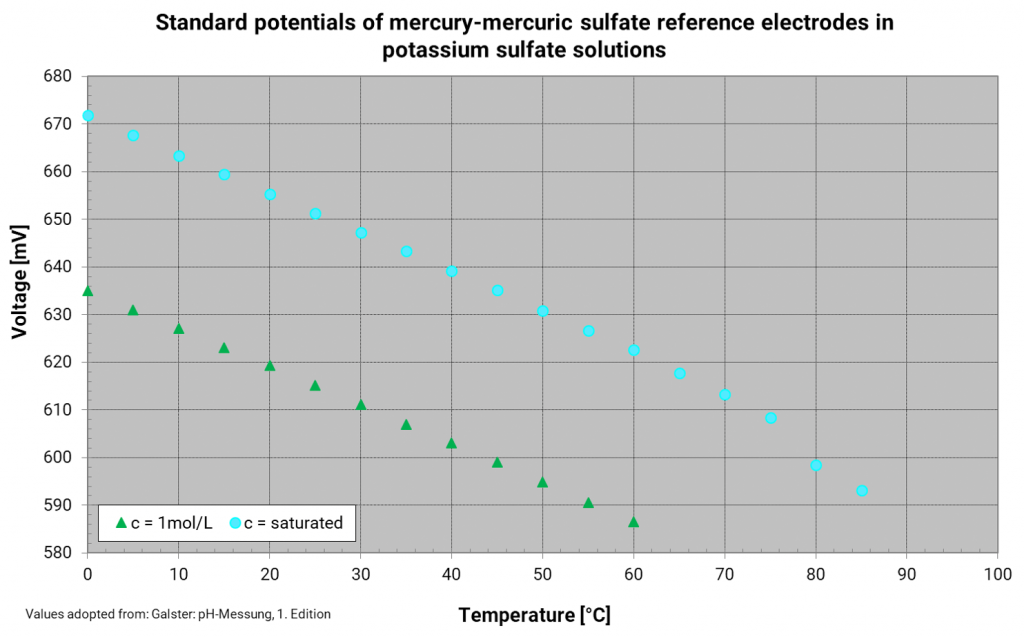
Mercury mercury sulfate electrode
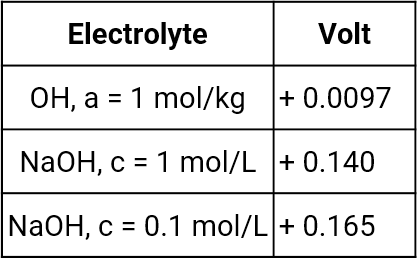
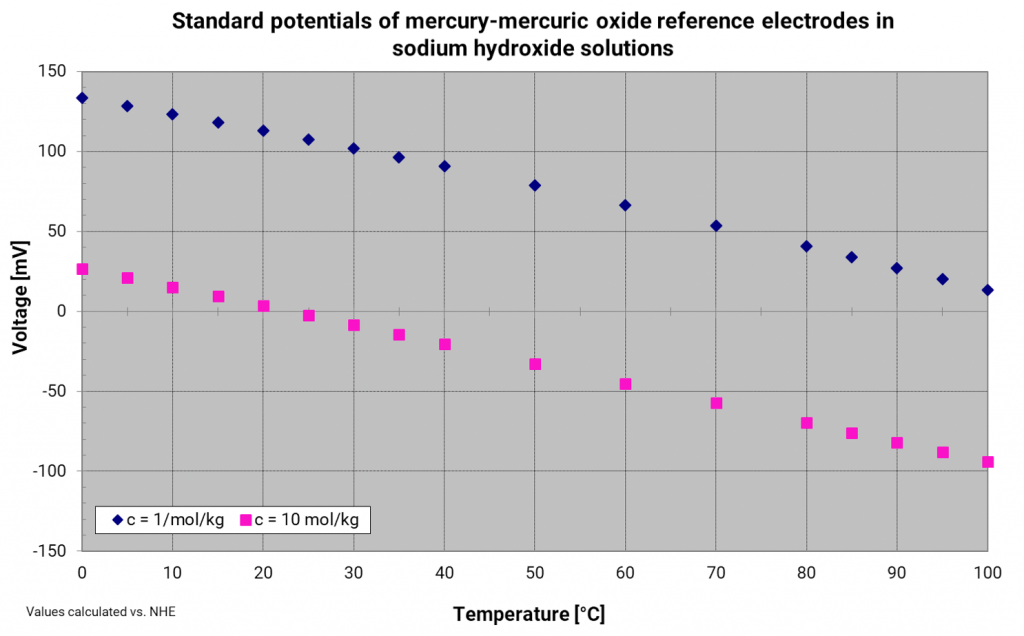
Mercury mercury oxide electrode
Electrolyte bridge – salt bridge
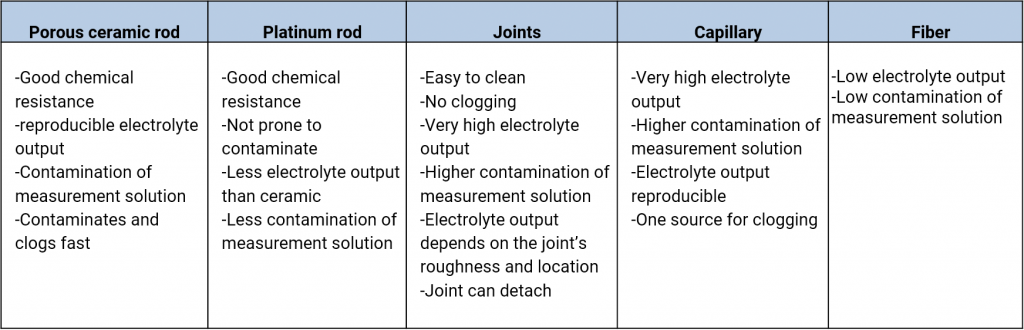
An electrolyte bridge, or salt bridge, is used for the contacting of various electrolytes. The diaphragm is here the ions conducting contact point. In the salt bridges of commercial reference electrodes one can find diaphragms made out of ceramics, platinum twine, grindings, slightest bores, capillaries such as the Haber-Luggin-Capillary, fibres, fabrics such as pellon or robust teflon.
The various materials have different rates of flow which depend on the porosity of the material and the size of the diaphragm. Also, the workmanship in the production of the reference electrode, damages, contaminations in the diaphragm can affect the rates of flow.
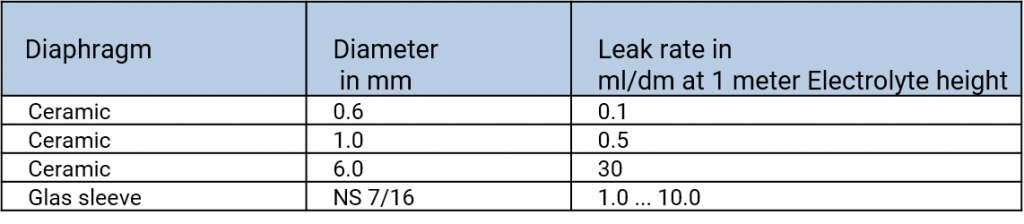
Rates of flow of various types of diaphragms, taken from Galster, pH-Messung, Wiley-VCH, 1990
When using electrolyte bridges, one has to observe that according to the type of the diaphragm different amounts of interior electrolyte flow out into the measuring solution. The choice of the reference electrode depends on the measuring solution in which the electrode should be used. To avoid contaminations of the interior electrolyte in the reference electrode caused by diffusing measuring solution, the effusion of the interior electrolyte into the measuring solution is necessary. Therefore, the filling hole should be open or at least not be closed airtight while the measurements. The filling level of the interior electrolyte should be significantly higher than the level of the measuring solution.
In difficult measured media such as highly contaminated or highly viscous samples ground diaphragms are preferably used because of their high rates of flow.
Reference electrodes with a solid interior electrolyte do not have any electrolyte outflow. Though, through the diaphragm the measuring solution can enter into the solid electrolyte and contaminate, dissolve or dilute it. Interior electrolyte out of the reference electrode contaminates your measuring solution. Reference electrodes have to be refilled repeatedly. The liquid level in the reference electrode must always be higher than the level of the measuring solution. Reference electrodes with a solid electrolyte become inaccurate over time because the solid electrolyte changes due to the entering ions of the measuring solution.
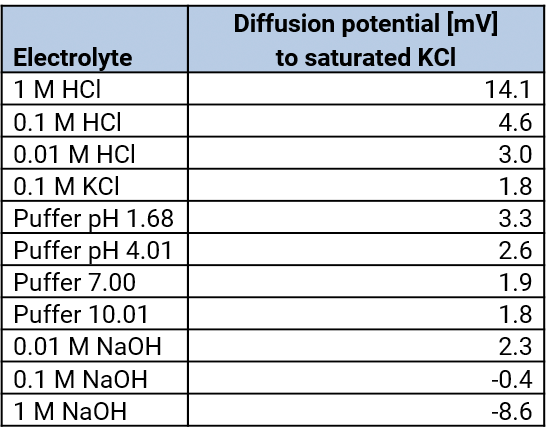
At every diaphragm a diffusion voltage arises which lead to deviations of the measured potential.
Diffusion voltage – diffusion potentials
The diaphragm is the contact point between the bridging electrolyte and the measuring electrolyte. At this point, the diffusion voltages arise because the anions and the cations of different electrolytes have different migration velocities. Diffusion voltages can be reduced by the choice of the bridging electrolyte and the kind of the salt bridge.





 Deutsch
Deutsch