Principles of electrochemical cells
What are electrochemical cells?
Electrochemical cells are systems which transfer energy of chemical reactions into electrical energy. Conversely, electrical energy can also be used to run electrochemical reactions. Typical electrochemical cells are batteries, accumulators, fuel cells or electrolytic cells. An electrochemical cell consists of two so-called half-cells whereby, each half-cell consists of an electrode which immerses in an electrolyte. Both half-cells can be immersed in the same electrolyte or in different electrolytes. Two half-cells in different electrolytes must be connected ionic via an electrolyte bridge or a salt bridge. The easiest variant is a filter paper soaked in KNO3 or KCI. Others are made of glass.
A special form of the salt bridge is the Haber-Luggin-Capillary. Every reference electrode with an interior electrolyte works with a salt bridge. By the way, every reference electrode is a half-cell. The chemical reactions in a half-cell can affect the electrolyte, the electrode or components supplied from outside such as hydrogen gas in fuel cells. Two half-cells which are connected ionic and electrically form a full cell. One of the half-cells emits electrons (oxidation). These electrons flow through the outer circuit into the second half-cell where appropriate reductions processes take place. The salt bridge allows a flow of anions and cations of the electrolyte so that an equilibrium between the half-cells can occur. Without this ionic connection the load difference, caused by the electron flow, would bring the reactions to a standstill.
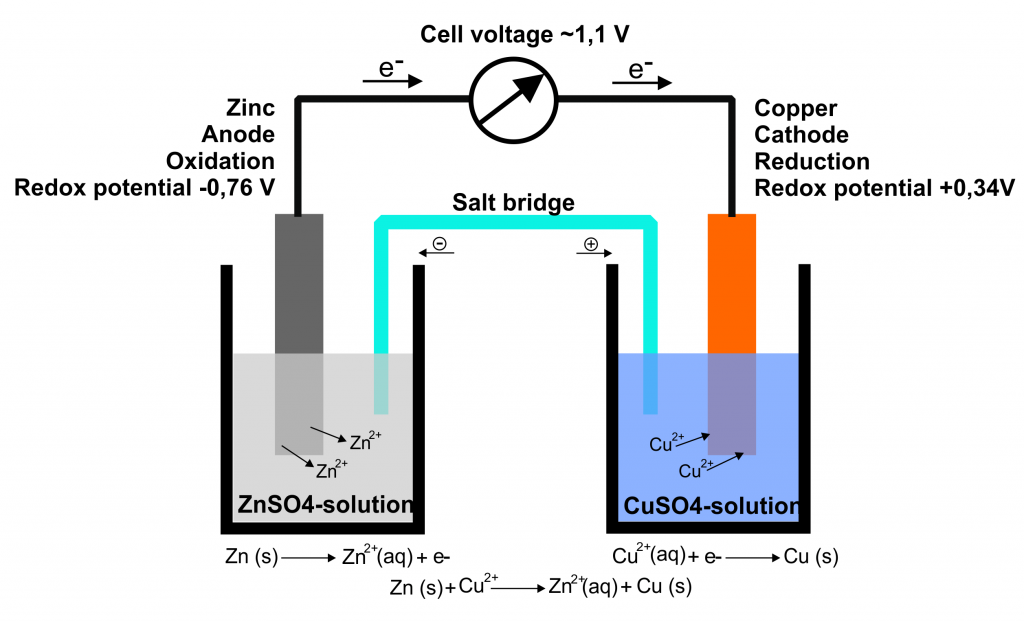
A typical half-cell which is known in the electrochemistry is the so-called Daniell cell. It consists of a zinc rod in zinc sulphate solution and a copper rod in copper sulphate solution. For the ion exchange, both half-cells are connected via a salt bridge. If a multimeter is interposed between the half-cells a cell voltage of ca. 1.1 Volt can be read off. On each half-cell (often called half-element) a potential sets which can be measured by means of a reference electrode.
A zinc and a copper half-cell are combined to a full cell, the Daniell cell
How do electrochemical potentials arise?
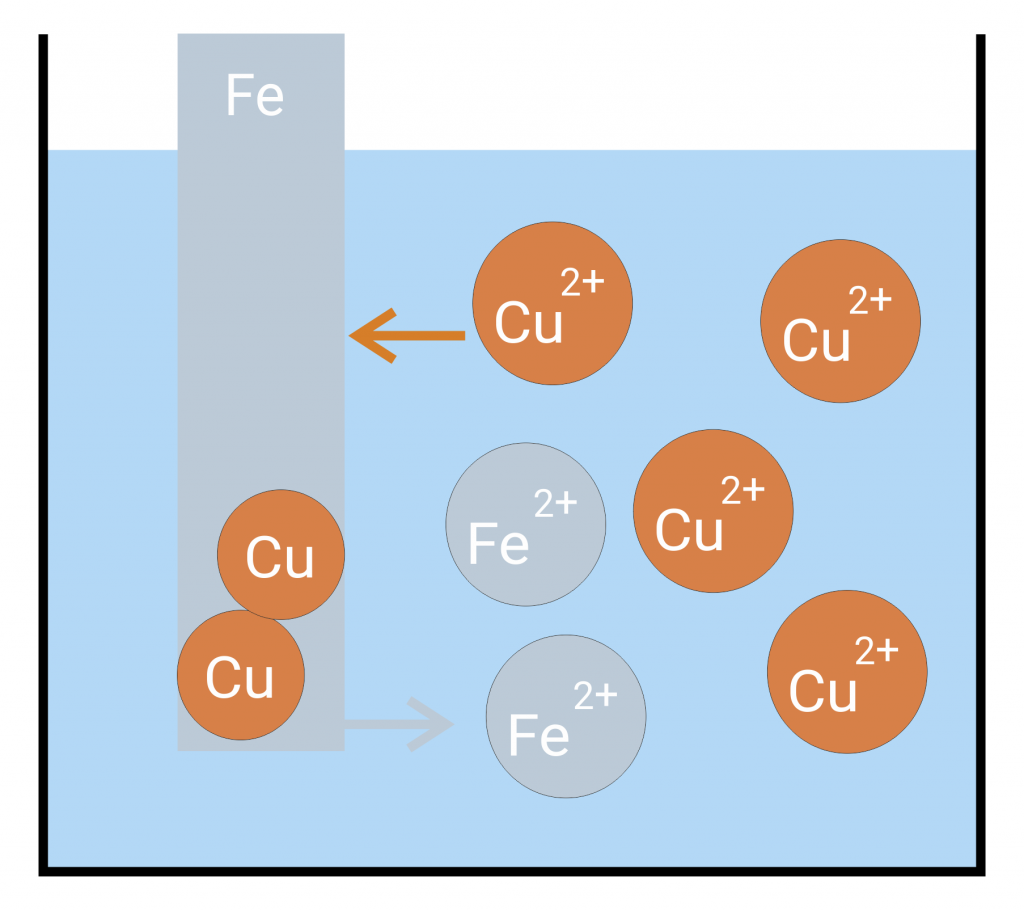
If a sheet iron is immersed in copper saline a copper precipitation is formed on the iron. If you reverse the experiment (immerse a sheet copper in ferrous saline) nothing happens.
How do these two metals differ from each other?
An equilibrium between the metal and the liquid arises when they are brought into contact. Metal ions dissolve out of the lattice of the metal which is immersed in a liquid of its own or other metal ions. That is known as solution pressure which is yielded by the difference of the lattice energy and the hydration energy. In return, metal ions are pressed back into the metal lattice by the separation pressure.
In the example above, that means that iron ions go into the solution while the copper ions present in the solution absorb the remaining electrons in the sheet iron and separate as copper on the sheet iron. If you reverse the experiment, neither copper goes into the solution nor iron ions are separated. Thus, the solution pressure of iron is higher than the solution pressure of copper.
If the solution pressure dominates an excess of electrons arises at the metal rod. If the separation pressure dominates a shortage of electrons arises at the metal rod. A dynamic equilibrium is formed. The separation of the metal ions is thereby supported by the electric attraction of the negatively charged metal. The solution pressure, the separation pressure and the electric pressure lead to a state of minimal energy in which electric work is done. The electric work can be seen in the form of voltage at the metal rod.
That absolute equilibrium voltage as such cannot be determined experimentally since only voltage differences are experimentally accessible. Therefore, a second electrode is needed which is called comparative electrode, reference electrode, indifferent electrode or indicator electrode. Its equilibrium voltage cannot be measured separately, too. To compare the voltages of different metals in their solutions they have to be measured against the same reference electrode. Therefore, you should use a reference electrode whose equilibrium voltage sets fast and reproducibly
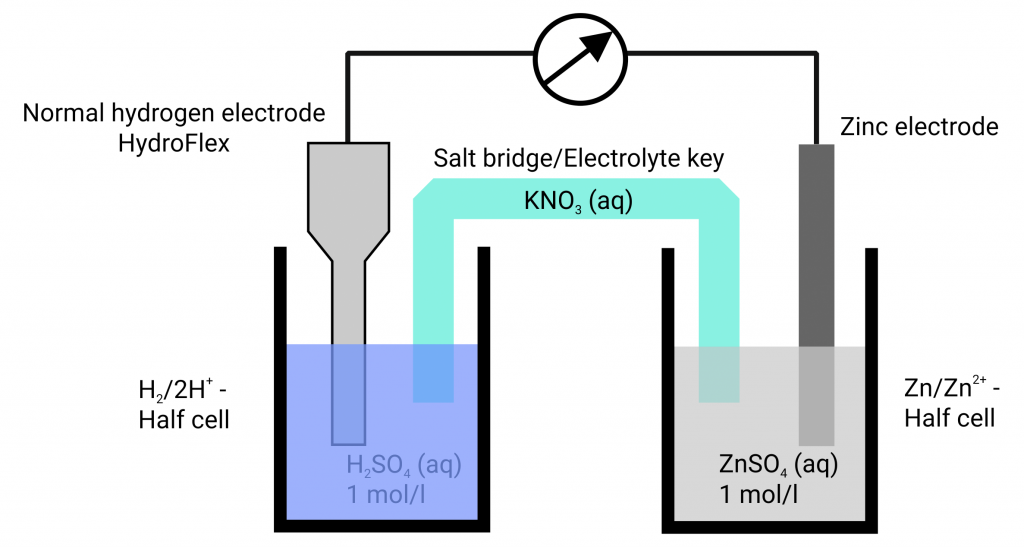
In the past, a platinum plated platinum electrode washed round with hydrogen gas in hydrochloric acid has established itself. The equilibrium voltage at standard conditions (hydronium ion activity of the hydrochloric acid 1 mol/l; hydrogen gas 1.013 bar) has been defined as 0.000 V at all temperatures. Only then, the hydrogen electrode is designated as a standard hydrogen electrode. If the voltage differences of various metals in their metal salt solutions are measured against the standard hydrogen electrode, different voltages arise which can be higher or lower than 0.000 V. The expected equilibrium potentials of different systems at standard conditions (25°C, metal ion activity 1 mol/l) are collated in the electrochemical series. Metals with an equilibrium potential lower than 0 V are designated as ignoble. If the equilibrium potentials are higher than 0 V the metals are designated as noble.
Reference electrode – but which one?
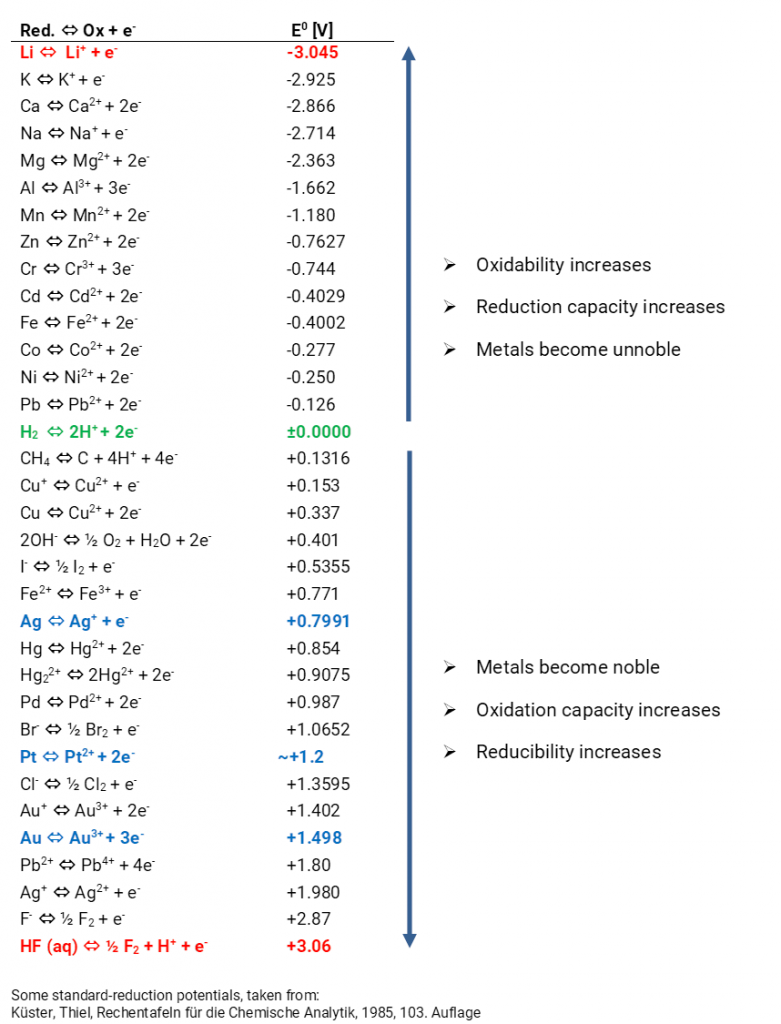
To measure electrochemical potentials you need your to study object as well as a second electrode with a known and constant potential, a so-called reference electrode. In that case, you have a simple two electrodes set-up and the measurement happens quasi currentless.
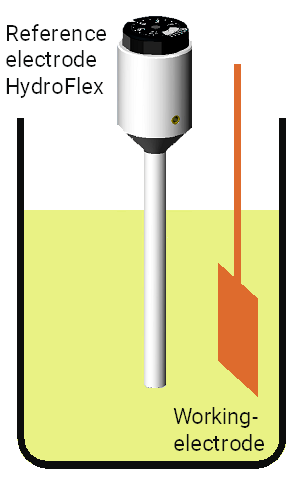
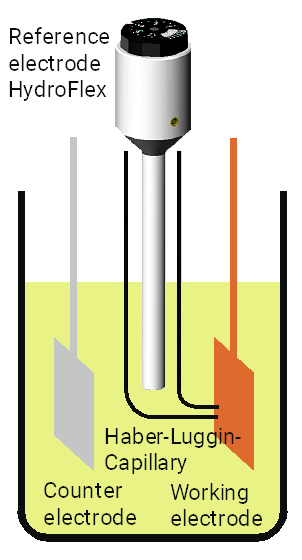
If you want to measure the potential and the current you have to choose a three electrodes set-up. The third electrode is the so-called counter electrode which is used to detect the electrical current.
In this set-up you must insert the reference electrode with a Haber-Luggin-Capillary to minimize the voltage drop between the working electrode and the counter electrode and to avoid disruptions which are caused by the current flowing between the working and the counter electrode.
In both cases it is important to choose the right reference electrode – a large number of reference electrodes is available. Most of them work with an interior electrolyte which contains chlorides such as potassium chloride. In that case you will contaminate your measuring solution with potassium chloride. That can cause big problems, especially if you want to study corrosion processes because chloride can strengthen the corrosion of your material. The best is to work with an electrolyte-free reference electrode (indicator electrode). We suggest the hydrogen reference electrode HydroFlex since it works without an interior electrolyte and every electrochemist should work with it. There are no mistakes caused by diffusion voltages.




 Deutsch
Deutsch