Usage of the hydrogen electrodes HydroFlex and Mini-HydroFlex
HydroFlex as RHE (reversible hydrogen electrode – indicator electrode)
The use of HydroFlex as RHE is the most common way of usage in the laboratory daily routine. Just place the HydroFlex in your measuring solution. The benefits are quite obvious. You do not need an electrolyte bridge; you do not have a diffusion potential und no contamination by ions flowing out of your reference system. HydroFlex is very useful for continuous measurements because it is – apart from the replacement of the hydrogen supply every 6 months – maintenance-free.
HydroFlex as NHE (normal hydrogen electrode)
If you place the hydrogen electrode in 1 mol/l hydrochloric acid at ambient pressure you get a normal hydrogen electrode. The potential variance of a NHE in comparison to a SHE is very low while the experimental effort is within limits.
HydroFlex as SHE (standard hydrogen electrode)
The standard hydrogen electrode is the most important reference electrode because its potential is defined as the zero point of the electrochemical series:
For solutions in protic solutions, the universal reference electrode for which, under standard conditions, the standard electrode potential H+/H2 is zero at all temperatures.“ (IUPAC Compendium of Chemical Terminology, Goldbook, Version 2.3.3., 24.03.2014).
Standard hydrogen electrode: „The standard hydrogen electrode consists of a platinum electrode in contact with a solution of H+ at unit activity and saturated with H2 gas with a fugacity referred to the standard pressure p∅ of 105 Pa. “ (Quantities, units and symbols in physical chemistry, IUPAC Green Book, 3rd edn, 2nd printing, IUPAC & RSC Publishing, Cambridge, 2008). Since 1982 the IUPAC specifies the standard pressure as 1.000 bar (100 kPa). Before 1982 the standard pressure was defined as 1.01325 bar (101.325 kPa = 1 atm). For this reason, you can often find the value of 1.01325 bar in credentials which is still used in the electrochemistry as the preferred standard.
To be used as a SHE the hydrogen electrode has to be placed in an electrolyte with a hydrogen ion activity of 1 mol/l (e.g. at 25°C a 1.18 molar hydrochloric acid has a hydrogen ion activity of 1 mol/l). The temperature has to be 298.15 K und the pressure has to be 1013 hPa (standard conditions). That application plays a subordinate role since the setting of the pressure and the temperature is very difficult.
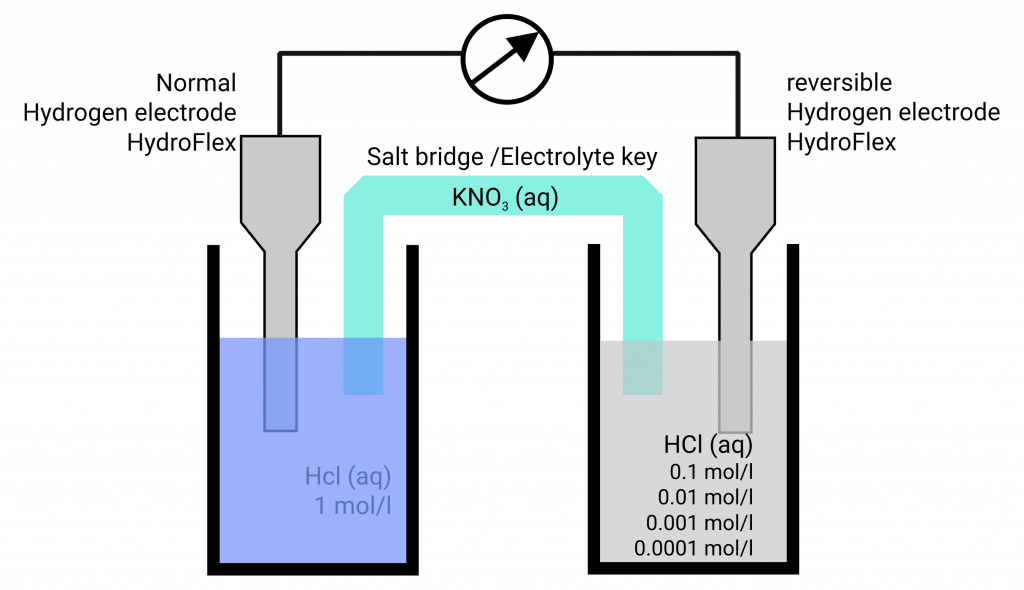
The hydrogen reference electrode can be used as a normal hydrogen electrode (left) and as a reversible hydrogen electrode (right)




 Deutsch
Deutsch