Gas diffusion electrodes
Gas diffusion electrodes from Gaskatel are porous electrodes for electrochemistry.
Various catalysts can be processed into electrodes.
The applications in electrochemistry are diverse and range from hydrogen electrodes
to electrodes for the reduction of carbon dioxide.
What are gas diffusion electrodes?
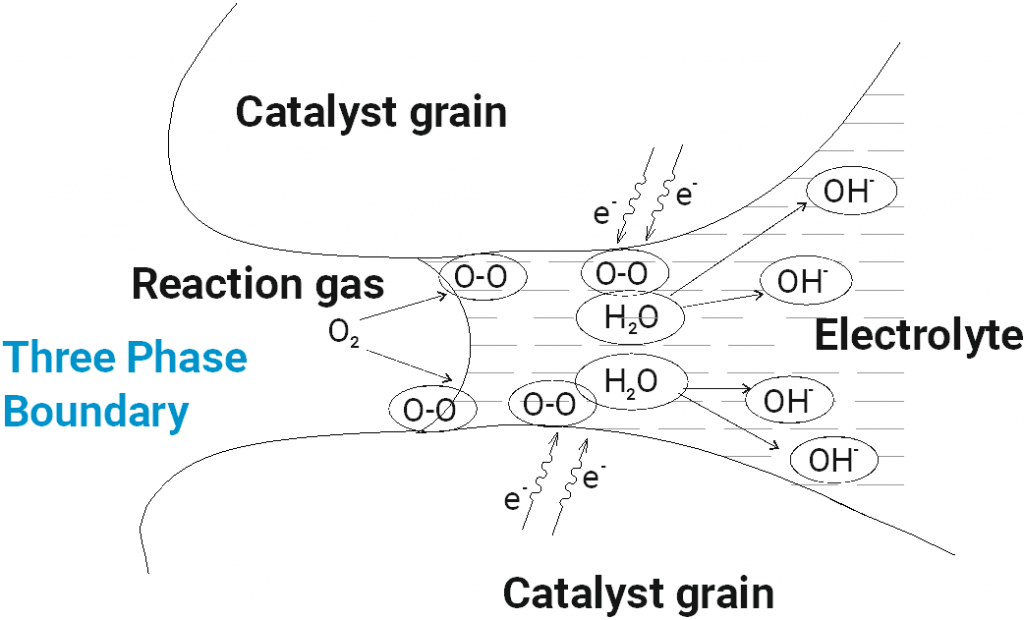
Gas diffusion electrodes are composed of a porous catalyst layer which has been applied on carrier material. The catalyst layer, which conducts electrons, catalyses an electrochemical reaction between the liquid and the gaseous phase. Thus, gas, electrolyte and the catalyst must be contacted. It is the so-called three-phase boundary on which three aggregate states (solid, liquid, gaseous) are contacted.
The electrode material as solid phase is the electron conductor and at the same time the catalyst that enables the actual reaction.
The electrolyte, which is usually liquid, is the ion conductor and reaction medium, providing soluble starting materials such as protons or hydroxide ions. At the same time, reaction products such as hydrogen peroxide or formate can also dissolve in the electrolyte. The charge-balancing ion transport takes place via the electrolyte.
The gaseous reaction partners involved in the reaction, such as hydrogen, air, oxygen or carbon dioxide, are in the gas phase.
The gases and the electrolyte must be brought into contact at the catalyst grains. Therefore, the electrode must have a porous structure. This requires a very large active electrode area with a small geometric surface. The starting materials must enter the pore system, and the reaction products must be transported out of the electrode. This also affects the water balance of the electrode. The higher the temperature, the faster these diffusion processes take place. Temperatures of 80-90 °C are optimal. Then gas diffusion electrodes are efficient and allow very high reaction rates.
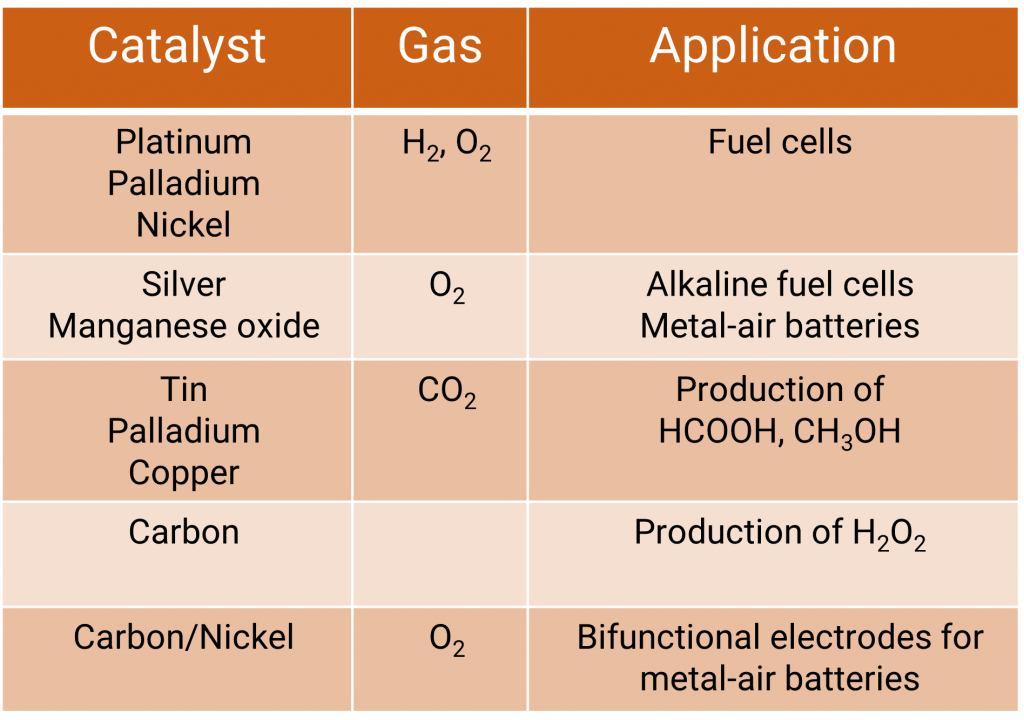
The production process allows the use of different catalysts:
- Platinum carbon
- Manganese mixed oxide/carbon
- Silver/silver carbon
- Nickel/nickel carbon
- Customer-specific catalysts
The catalyst layers are applied on woven metal nettings or carbon fleeces. They can be combined with porous PTFE-foil and/or microporous separators. Thus, we are able to develop and produce gas diffusion electrodes adjusted according to customer requests.
The BiPlex gas diffusion electrode is characterized by its porous electrode structure.
Typical fields of application are:
- Water electrolysis
- Chlorine alkali electrolysis
- Zinc air batteries
- Production of H2O2
- Wastewater treatment
- Double layer capacitor
Gas diffusion electrodes from Gaskatel
This nickel electrode is the most versatile of Gaskatel’s gas diffusion electrodes.
Gaskatel has a specially developed process so that the catalyst can be processed safely. This nickel catalyst is processed in this electrode. This nickel electrode is suitable for use in alkaline media.
The operating temperature is between 0°C and 100°C – just in the case of HOR the temperature should not be below 50°C.
The nickel electrode can be used in oxygen evolution.
Furthermore, the nickel electrode is a hydrogen electrode, it can be used in hydrogen evolution as well as in hydrogen consumption. For optimal performance, the nickel electrode should be activated before the measurements in hydrogen consumption, e.g. by operating it for 24 hours in the hydrogen stream at at least 50°C with a small reduction current.
Note that in hydrogen consumption the potential of the electrode must not rise above 150 mV vs. RHE, otherwise the catalyst may oxidise and become inactive.
The MOC-electrode is an oxygen consumption electrode, based manganese oxide and carbon.
anganese Dioxide and activated carbon support the 4 electron step – no peroxide formation will take place.
It can be operated in alkaline media in air and oxygen.
The OxAg is an oxygen consuming electrode.
The electrode is based on silver oxide. During operation as the cathode the silver oxide will be reduced and convert to silver – you should apply oxygen or air during that electrochemical reduction process otherwise the pore system is flooded by underpressure.It can be operated in alkaline media in air and oxygen.
The carbon electrode PerOx is an oxygen electrode, which is used in oxygen consumption as well as for hydrogen peroxide production.
It can be operated in alkaline media in air and oxygen.
1 mol/l KOH is the preferred electrolyte.
Electrode Tin
Tin electrodes were developed specifically for the conversion of carbon dioxide into products such as formate. The formate or similar reaction products are then further converted in other processes, sometimes with bacterial support. The operating parameters are also determined by the processes that follow the conversion.
Therefore, the electrode must always be adapted accordingly and is available on request.
Electrode Copper
Copper electrodes are also used in the electrochemical reduction of carbon dioxide. They are operated in a neutral solution between pH 4 and pH 12.
This electrode is available on request.
Bifunctional air electrode OrRev
A special feature is the so-called bifunctional air electrode, specially developed for metal-air batteries. These electrodes can produce oxygen as well as consume oxygen. With these electrodes, compact metal-air batteries can be realised in alkaline media.
This electrode is available on request.



 Deutsch
Deutsch