ElyFlow
test cell optimized for electrolyte circuits
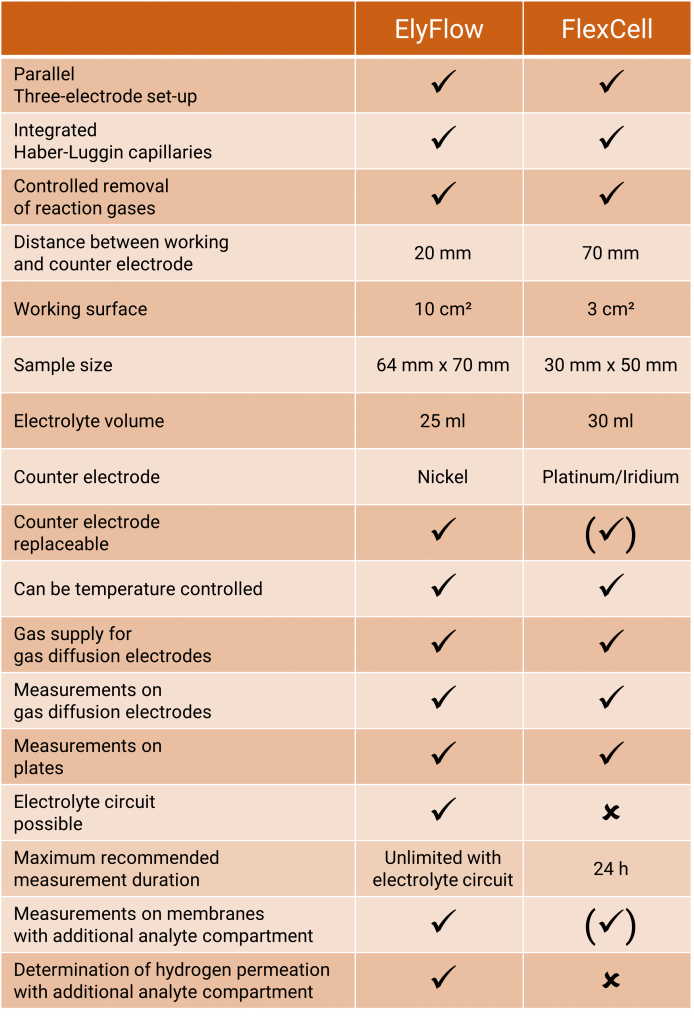
ElyFlow is a test cell optimized for electrolyte circuits for electrochemistry with a three-electrode set-up. Working electrode and counter electrode are arranged in parallel. The reference electrode, preferably a Mini-HydroFlex, is located in a separate reservoir. The potential is measured via Haber-Luggin capillaries that end directly at a fixed distance from the working electrode.
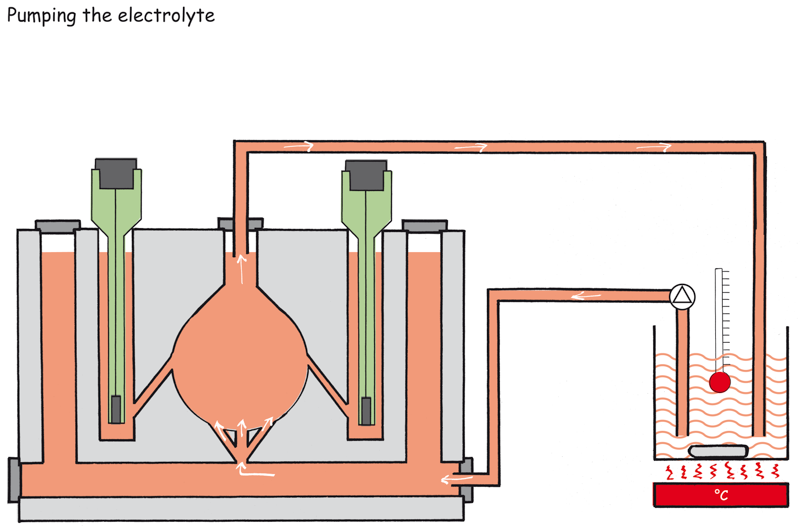
In addition, the electrolyte can be pumped through the cell.
The newly developed measuring cell from Gaskatel offers completely new possibilities for your electrochemical measurements.
It is optimized for measurements with an electrolyte circuit.
You can use it to characterize your plates and gas diffusion electrodes as usual.
Investigations on membranes are also possible with it.
Hydrogen permeation is your topic? No problem – this can also be investigated with ElyFlow.
Like FlexCell, ElyFlow is also flexible regarding to the sample (e.g. metal sheet, foils, gas diffusion electrode) – but rigid where it really counts:
- Fixed and thus defined distance between the working and the counter electrode
- Fixed and thus defined distance between the working and the reference electrode
ElyFlow chemical resistant and indestructible since it is made of Polytetrafluorethylene (PTFE).
ElyFlow can be used in a pH range of pH 8 to pH 16.
The electrolyte volume is only 25 ml.

Temperature control box for test cell ElyFlow
The test cell can be heated up to 100°C by an integrated PTC heating element. The heating element is mounted behind the counter electrode holder. The contact is made via 4 mm banana sockets. The temperature control takes place via a Pt100-probe. For temperature control, use the temperature control box from Gaskatel.
The test cell can be heated up to 100°C by an integrated PTC heating element. The heating element is mounted behind the counter electrode holder. Contact is made via 4 mm banana sockets.
To give your electrochemical measurements even more control, we have further developed the temperature control box into a control box.
You can heat one chamber of your measuring cell as usual.
In addition, you can now pump the electrolyte through the cell. There are 2 electrolyte pumps and control units available.
If you are investigating gas diffusion electrodes, you can measure the pressure.
A galvanostat and a potential measurement allow you to make simple electrochemical measurements without the need for a potentiostat.
The control box can be controlled via software and the measurement signals can be tapped via an RS 485 interface.

Control box TP for more control of your electrochemical measurements.
Manufactured of plastic
ElyFlow test cell is made of polytetrafluoroethylene (PTFE). With the PTFE test cell, you can even measure in fluoride-containing and strongly alkaline media.
ElyFlow is insensitive to breakage and virtually indestructible.
CNC technology enables precise and reproducible production of all relevant bores, recesses for seals, etc.
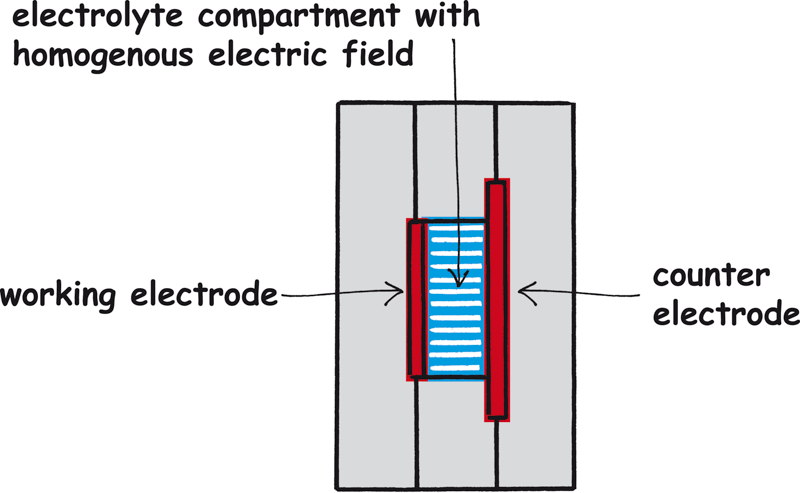
Homogeneous electrical field
Homogeneous electrical field
Working and counter electrodes are arranged in parallel, as with FlexCell.
The electrolyte chamber is tubular. This also ensures a homogeneous electric field in ElyFlow.
Electrode size – electrode distance
The working surface of the electrode is 10 cm².
The distance between the working electrode and the counter electrode is
2 cm. Measurements in poorly conducting electrolytes are possible now.
Higher current densities can be impressed.
Haber-Luggin capillaries
There are two small reservoirs for the reference electrode, which merge into immobile Haber-Luggin capillaries.
The Haber-Luggin capillaries ends close to the working surface. This means that the potential is not disturbed by the streamline field or by any gas bubbles that are generated. The voltage drop across the electrolyte is very low due to the close distance.
The CNC-technology in plastic allows the precise positioning of the Haber-Luggin Capillary to the working electrode. That ensures the comparability of the measurements among each other and between the test cell.
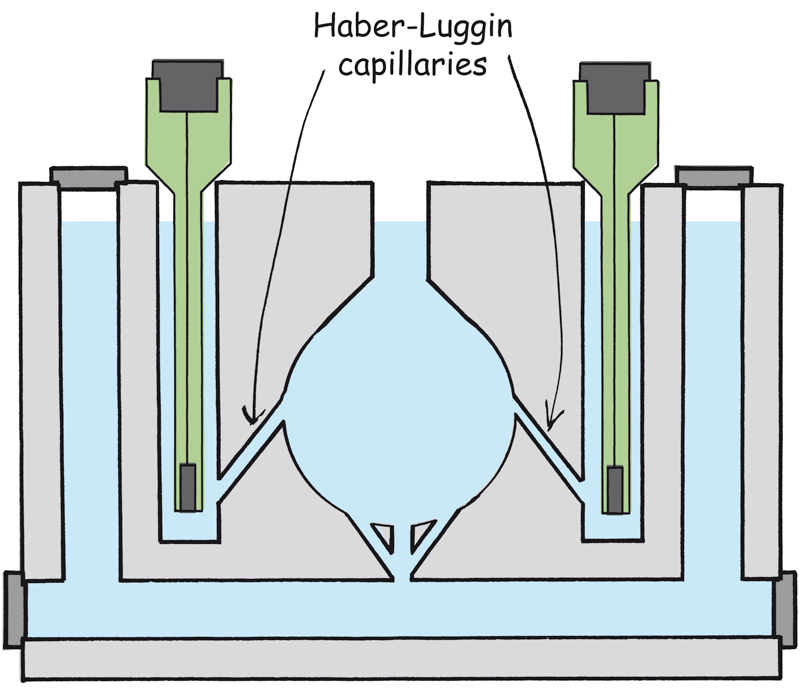
Reservoirs and Haber-Luggin capillaries for the reference electrodes in test cell Elyflow
Electrolyte distribution and removal of gas bubbles
There is an electrolyte inlet with three openings on each side, which distributes the electrolyte flow towards the working and counter electrodes.
At the electrolyte outlet there is a cavity (dome) for collecting the electrolyte and the controlled removal of gas bubbles from the electrochemical process.
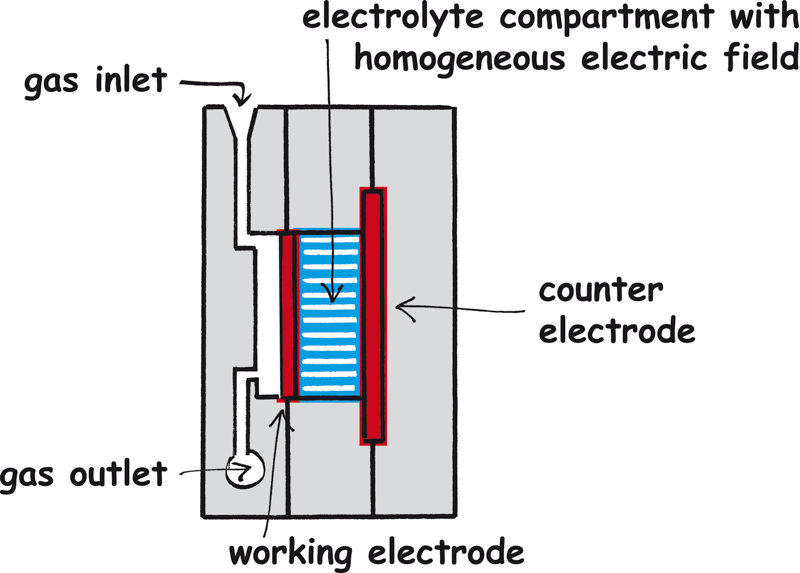
Gas supply of gas diffusion electrodes
Gas diffusion electrodes can be supplied with reaction gases via the connections on the gas compartment. It is best to use a needle valve to control the gas flow.
Electrolyte volume and electrolyte analysis
The electrolyte volume in the cell is 25 ml.
However, it can be increased by pumping the electrolyte from a storage vessel through the measuring cell with the aid of a diaphragm pump. In this way, longer measuring times are possible.
In addition, the electrolyte in the storage vessel can be continuously monitored during the measurement: temperature, conductivity, pH-value, analysis of reaction products.
Use a heatable storage vessel when measuring at higher temperatures.
Protection from unwanted by-products
Often the counter electrode is the anode. This means that very oxidizing ions are generated there. Depending on the electrolyte, these can be peroxides, perchlorate, persulfates, etc. Even the smallest amount of these ions reaching the working electrode can trigger severe corrosion processes.
With the additional analyte compartment for ElyFlow, a membrane can be installed to protect the working electrode from the reaction products.
Applications of ElyFlow in electrochemistry
Electroplating
With an electrolyte circuit, controlled electroplating is possible because the electrolyte is constantly renewed. Thus, a depletion with reactants in front of the working electrode is excluded.
CO2 reduction
The electrochemical conversion of CO2 into formates or carbonates is highly topical.
ElyFlow enables the electrolyte to be renewed continuously. In the storage vessel, for example, temperature, pH value or conductivity can be monitored. In addition, an analysis of the reaction products can be carried out with the appropriate sensor technology.
Determination of membrane resistance
Since each analyte compartment has Haber Luggin capillaries, you can use two analyte compartments to measure the membrane resistance.
To measure the voltage, drop across the membrane, both analyte spaces must be positioned so that the Haber Luggin capillaries face the membrane.
The voltage drop across the membrane is taken as the difference between the two reference electrodes inside the Haber-Luggin capillaries.
During your measurements with electrochemical cells typical problems are caused by:
Corroded contacts and measuring cables
Check the measuring cables if there are any visual damages such as corrosion, cracks or sessile plugs. Replace the cables.
Reference electrode
Gas bubbles in the Haber-Luggin-Capillary or air bubbles in the inner electrolyte of the reference electrode can disturb the potential measurements. Diffusion voltages caused by the diaphragm of the reference electrode can cause measuring errors.
Electrolyte film
An electrolyte film between the reference electrode and the counter electrode can lead to a short.
Contaminations
Contaminations, degradation products, corrosion products can lead to incorrect measurements.
Battery status
An infirm battery of handheld multimeter lead to wrong voltages.
Potentiostat and filter
The potentiostat often reacts very delicate to electrolytes and/or samples with inadequate conductivity or gas bubbles in the Haber-Luggin-Capillary. It begins to oscillate. You can find more helpful information here What-can-cause-my-experiment-to-be-noisy.
Software
Pay attention to the correct input of the signs of your measurement parameters in the software. Bugs in the software are also possible.





 Deutsch
Deutsch